PPARγ agonist treatment reduces fibroadipose tissue in secondary lymphedema by exhausting fibroadipogenic PDGFRα+ mesenchymal cells
- PMID: 38131378
- PMCID: PMC10807713
- DOI: 10.1172/jci.insight.165324
PPARγ agonist treatment reduces fibroadipose tissue in secondary lymphedema by exhausting fibroadipogenic PDGFRα+ mesenchymal cells
Abstract
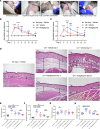
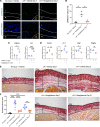
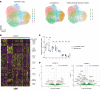
Secondary lymphedema occurs in up to 20% of patients after lymphadenectomy performed for the surgical management of tumors involving the breast, prostate, uterus, and skin. Patients develop progressive edema of the affected extremity due to retention of protein-rich lymphatic fluid. Despite compression therapy, patients progress to chronic lymphedema in which noncompressible fibrosis and adipose tissue are deposited within the extremity. The presence of fibrosis led to our hypothesis that rosiglitazone, a PPARγ agonist that inhibits fibrosis, would reduce fibrosis in a mouse model of secondary lymphedema after hind limb lymphadenectomy. In vivo, rosiglitazone reduced fibrosis in the hind limb after lymphadenectomy. Our findings verified that rosiglitazone reestablished the adipogenic features of TGF-β1-treated mesenchymal cells in vitro. Despite this, rosiglitazone led to a reduction in adipose tissue deposition. Single-cell RNA-Seq data obtained from human tissues and flow cytometric and histological evaluation of mouse tissues demonstrated increased presence of PDGFRα+ cells in lymphedema; human tissue analysis verified these cells have the capacity for adipogenic and fibrogenic differentiation. Upon treatment with rosiglitazone, we noted a reduction in the overall quantity of PDGFRα+ cells and LipidTOX+ cells. Our findings provide a framework for treating secondary lymphedema as a condition of fibrosis and adipose tissue deposition, both of which, paradoxically, can be prevented with a pro-adipogenic agent.
Keywords: Adipose tissue; Cell Biology; Fibrosis.
Conflict of interest statement
Figures

References
-
- Giuliano AE, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. - DOI - PMC - PubMed
-
- Giuliano AE, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the american college of surgeons oncology group (alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264(3):413–420. doi: 10.1097/SLA.0000000000001863. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

