Clinical Long-Term Outcomes of Patient-Reported Outcomes in the Prospective Real-World Tofacitinib Response in Ulcerative Colitis Registry
- PMID: 38131617
- PMCID: PMC10962890
- DOI: 10.14309/ctg.0000000000000669
Clinical Long-Term Outcomes of Patient-Reported Outcomes in the Prospective Real-World Tofacitinib Response in Ulcerative Colitis Registry
Abstract
Introduction: We previously reported the results of tofacitinib induction therapy in the prospective multisite US real-world Tofacitinib Response in Ulcerative Colitis registry. We now assessed patient-reported outcomes (PROs) and predictors of success during tofacitinib maintenance therapy.
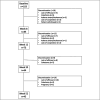
Methods: Tofacitinib Response in Ulcerative Colitis included 103 patients with refractory ulcerative colitis (UC); 67% had failed ≥ 2 biologics. Patients reported the Simple Clinical Colitis Activity Index (SCCAI), Patient-Reported Outcome Measurement Information System measures for anxiety, depression, social satisfaction, and adverse events between weeks 8 and 52 using a web-based system. Paired t test and P for trend were used to compare changes in PRO measures over time. Bivariate analyses and logistic regression models were used to determine factors associated with response (SCCAI <5) or remission (SCCAI <2) at week 52.
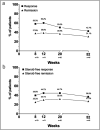
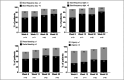
Results: Of 103 patients, 82.5% entered the maintenance phase and 43.7% remained on tofacitinib at week 52. Tofacitinib de-escalation to 5 mg BID occurred in 15% of patients. At week 52, 42.7% and 31.1% of all patients reported an SCCAI <5 and SCCAI ≤2, respectively. Normalization of bowel frequency, rectal bleeding, and urgency occurred in 79%, 61%, and 48% of patients remaining on maintenance therapy. Social satisfaction improved significantly ( P < 0.001), while anxiety and depression scores only numerically improved. No consistent predictors for tofacitinib long-term treatment efficacy were identified, and safety findings were consistent with the known safety profile of tofacitinib.
Discussion: Tofacitinib is an effective maintenance therapy in patients with refractory UC. Dose reductions infrequently occurred during maintenance. Unmet needs in UC maintenance include improvement of urgency and psychosocial factors (NCT03772145).
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

References
-
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: Ulcerative colitis in adults. Am J Gastroenterol 2019;114(3):384–413. - PubMed
-
- Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376(18):1723–36. - PubMed
-
- Sandborn WJ, Panes J, D'Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019;17(8):1541–50. - PubMed
-
- Kayal M, Posner H, Spencer E, et al. Net remission rates with biologic and small molecule treatment in ulcerative colitis: A reappraisal of the clinical trial data. Clin Gastroenterol Hepatol 2023;21(13):3433–6.e1. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

