Tiny swabs: nasal swabs integrated into tube caps facilitate large-scale self-collected SARS-CoV-2 testing
- PMID: 38131692
- PMCID: PMC10865831
- DOI: 10.1128/jcm.01285-23
Tiny swabs: nasal swabs integrated into tube caps facilitate large-scale self-collected SARS-CoV-2 testing
Abstract
The COVID-19 pandemic spurred the development of innovative solutions for specimen collection and molecular detection for large-scale community testing. Among these developments is the RHINOstic nasal swab, a plastic anterior nares swab built into the cap of a standard matrix tube that facilitates automated processing of up to 96 specimens at a time. In a study of unsupervised self-collection utilizing these swabs, we demonstrate comparable analytic performance and shipping stability compared to traditional anterior nares swabs, as well as significant improvements in laboratory processing efficiency. The use of these swabs may allow laboratories to accommodate large numbers of sample collections during periods of high testing demand. Automation-friendly nasal swabs are an important tool for high-throughput processing of samples that may be adopted in response to future respiratory viral pandemics.
Keywords: respiratory pathogens; scaled testing; specimen collection.
Conflict of interest statement
The authors have no relationship with the Rhinostics corporation. Dr. Chu reported consulting with Ellume, Pfizer, and the Bill and Melinda Gates Foundation. She has served on advisory boards for Vir, Merck, and Abbvie. She has conducted CME teaching with Medscape, Vindico, Catalyst CME, and Clinical Care Options. She has received support and reagents from Ellume and Cepheid outside of the submitted work. Dr. Englund reports consulting with AstraZeneca, Meissa Vaccines, Moderna, Pfizer, and Sanofi Pasteur, and research support from AstraZeneca, GlaxoSmithKline, Merck, Moderna, and Pfizer.
Figures


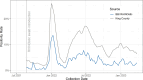
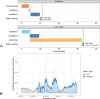
References
-
- McCulloch DJ, Kim AE, Wilcox NC, Logue JK, Greninger AL, Englund JA, Chu HY. 2020. Comparison of unsupervised home self-collected midnasal swabs with clinician-collected nasopharyngeal swabs for detection of SARS-CoV-2 infection. JAMA Netw Open 3:e2016382. doi:10.1001/jamanetworkopen.2020.16382 - DOI - PMC - PubMed
-
- Tu YP, Jennings R, Hart B, Cangelosi GA, Wood RC, Wehber K, Verma P, Vojta D, Berke EM. 2020. Patient-collected tongue, nasal, and mid-Turbinate Swabs for SARS-Cov-2 yield equivalent sensitivity to health care worker collected nasopharyngeal Swabs. Infectious diseases (except HIV/AIDS). doi:10.1101/2020.04.01.20050005 - DOI
-
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, REDCap Consortium . 2019. The REDcap consortium: building an international community of software platform partners. J Biomed Inform 95:103208. doi:10.1016/j.jbi.2019.103208 - DOI - PMC - PubMed
-
- Srivatsan S, Heidl S, Pfau B, Martin BK, Han PD, Zhong W, van Raay K, McDermot E, Opsahl J, Gamboa L, Smith N, Truong M, Cho S, Barrow KA, Rich LM, Stone J, Wolf CR, McCulloch DJ, Kim AE, et al. , Seattle Flu Study Investigators . 2021. Swabexpress: an end-to-end protocol for extraction-free COVID-19 testing. Clin Chem 68:143–152. doi:10.1093/clinchem/hvab132 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

