Assessing Ethnic Minority Representation in Fibromyalgia Clinical Trials: A Systematic Review of Recruitment Demographics
- PMID: 38131736
- PMCID: PMC10742509
- DOI: 10.3390/ijerph20247185
Assessing Ethnic Minority Representation in Fibromyalgia Clinical Trials: A Systematic Review of Recruitment Demographics
Abstract
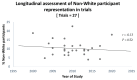
The under-representation of non-White participants in Western countries in clinical research has received increased attention, due to recognized physiological differences between ethnic groups, which may affect the efficacy and optimal dosage of some treatments. This review assessed ethnic diversity in pharmaceutical trials for fibromyalgia, a poorly understood chronic pain disorder. We also investigated longitudinal change to non-White participant proportions in trials and non-White participants' likelihood to discontinue with fibromyalgia research between trial stages (retention). First, we identified relevant trials conducted in the United States and Canada between 2000 and 2022, by searching PubMed, Web of Science, Scopus, and the Cochrane Library databases. In trials conducted both across the United States and Canada, and exclusively within the United States, approximately 90% of participants were White. A longitudinal analysis also found no change in the proportion of non-White participants in trials conducted across the United States and Canada between 2000 and 2022. Finally, we found no significant differences in trial retention between White and non-White participants. This review highlights the low numbers of ethnic minorities in fibromyalgia trials conducted in the United States and Canada, with no change to these proportions over the past 22 years. Furthermore, non-White participants were not more likely to discontinue with the fibromyalgia research once they were recruited.
Keywords: ethnic minorities; fibromyalgia; longitudinal; non-White; pharmaceutical; retention; trials; underrepresentation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults.Cochrane Database Syst Rev. 2017 Oct 9;10(10):CD012172. doi: 10.1002/14651858.CD012172.pub2. Cochrane Database Syst Rev. 2017. PMID: 28990665 Free PMC article.
-
Targeted mass media interventions promoting healthy behaviours to reduce risk of non-communicable diseases in adult, ethnic minorities.Cochrane Database Syst Rev. 2017 Feb 17;2(2):CD011683. doi: 10.1002/14651858.CD011683.pub2. Cochrane Database Syst Rev. 2017. PMID: 28211056 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Antipsychotics for fibromyalgia in adults.Cochrane Database Syst Rev. 2016 Jun 2;2016(6):CD011804. doi: 10.1002/14651858.CD011804.pub2. Cochrane Database Syst Rev. 2016. PMID: 27251337 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Clinical Trial Participation Motivation: Role of Smoking Status.Healthcare (Basel). 2025 Feb 11;13(4):389. doi: 10.3390/healthcare13040389. Healthcare (Basel). 2025. PMID: 39997264 Free PMC article.
-
Community leaders' perceptions of pain and research engagement: Implications for participant diversity and inclusion in pain clinical trials.J Pain. 2025 Apr 8:105389. doi: 10.1016/j.jpain.2025.105389. Online ahead of print. J Pain. 2025. PMID: 40209867
-
Anti-inflammatory Role of Trypsin, Rutoside, and Bromelain Combination in Temporomandibular Joint Osteoarthritis: A Systematic Review.Cureus. 2024 Jan 6;16(1):e51749. doi: 10.7759/cureus.51749. eCollection 2024 Jan. Cureus. 2024. PMID: 38322061 Free PMC article. Review.
-
Age, Race, Ethnicity, and Sex of Participants in Clinical Trials Focused on Chronic Pain.J Pain. 2024 Aug;25(8):104511. doi: 10.1016/j.jpain.2024.03.007. Epub 2024 Mar 16. J Pain. 2024. PMID: 38492711 Free PMC article.
-
Milnacipran and Vanillin Alleviate Fibromyalgia-Associated Depression in Reserpine-Induced Rat Model: Role of Wnt/β-Catenin Signaling.Mol Neurobiol. 2025 Jun;62(6):7682-7705. doi: 10.1007/s12035-025-04723-w. Epub 2025 Feb 10. Mol Neurobiol. 2025. PMID: 39924579 Free PMC article.
References
-
- Commissioner O of the FDASIA Section 907: Inclusion of Demographic Subgroups in Clinical Trials. FDA. [(accessed on 29 April 2023)];2019 Available online: https://www.fda.gov/regulatory-information/food-and-drug-administration-....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

