Bictegravir/Tenofovir Alafenamide/Emtricitabine: A Real-Life Experience in People Living with HIV (PLWH)
- PMID: 38131882
- PMCID: PMC10742537
- DOI: 10.3390/idr15060069
Bictegravir/Tenofovir Alafenamide/Emtricitabine: A Real-Life Experience in People Living with HIV (PLWH)
Abstract
Background: Bictegravir (BIC), a recently introduced integrase inhibitor, is available in a single tablet regimen with tenofovir alafenamide (TAF) and emtricitabine (FTC) (BIC-STR). This study aimed to describe a real-life experience with BIC-STR.
Methods: We retrospectively analyzed the data of people living with HIV (PLWH) on antiretroviral therapy (ART) with BIC-STR followed by the Clinic of Infectious Diseases of Perugia (Perugia, Italy) from September 2019 to February 2023.
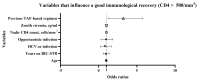
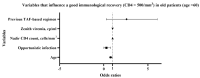
Results: 270 PLWH were enrolled with a median follow-up time on BIC-STR of 2.2 years (IQR 1.2-2.7). In the overall population, in treatment-experienced (N = 242), in treatment-naïve (N = 28), and in population with age > 60 years old (N = 86), we observed that CD4 cell count improved in absolute number, percentage and CD4/CD8 ratio, under BIC-STR. Patients with viremia < 50 cp/mL increased in all groups. In the overall population, previous ART with TAF and nadir CD4 cell count favored immunological recovery. In the ART-experienced group, time in therapy with BIC-STR was associated with HIV-RNA undetectability. In the older group, previous opportunistic infection and advanced age were associated with lower CD4 count.
Conclusions: BIC-STR was demonstrated, in real-life, to be a valid option for a switch, such as initial ART.
Keywords: HIV; antiretroviral; bictegravir; integrase inhibitors; real-life.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dąbrowska M.M., Wiercińska-Drapało A. Integrase inhibitors as a new class of ARV treatment. HIV AIDS Rev. 2007;6:10–14. doi: 10.1016/S1730-1270(10)60053-7. - DOI
-
- Lagi F., Botta A., Ciccullo A., Picarelli C., Fabbiani M., di Giambenedetto S., Borghi V., Mussini C., Bartoloni A., Sterrantino G. Early discontinuation of DTG/ABC/3TC and BIC/TAF/FTC single-tablet regimens: A real-life multicenter cohort study. HIV Res. Clin. Pract. 2021;22:96–101. - PubMed
-
- Mazzitelli M., Trunfio M., Putaggio C., Sasset L., Leoni D., Lo Menzo S., Mengato D., Cattelan A.M. Viro-Immunological, Clinical Outcomes and Costs of Switching to BIC/TAF/FTC in a Cohort of People Living with HIV: A 48-Week Prospective Analysis. Biomedicines. 2022;10:1823. doi: 10.3390/biomedicines10081823. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

