Injectable Thermoresponsive Microparticle/Hydrogel System with Superparamagnetic Nanoparticles for Drug Release and Magnetic Hyperthermia Applications
- PMID: 38131968
- PMCID: PMC10742759
- DOI: 10.3390/gels9120982
Injectable Thermoresponsive Microparticle/Hydrogel System with Superparamagnetic Nanoparticles for Drug Release and Magnetic Hyperthermia Applications
Abstract
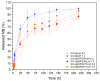
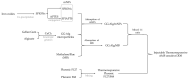
Cancer is a disease that continues to greatly impact our society. Developing new and more personalized treatment options is crucial to decreasing the cancer burden. In this study, we combined magnetic polysaccharide microparticles with a Pluronic thermoresponsive hydrogel to develop a multifunctional, injectable drug delivery system (DDS) for magnetic hyperthermia applications. Gellan gum and alginate microparticles were loaded with superparamagnetic iron oxide nanoparticles (SPIONs) with and without coating. The magnetic microparticles' registered temperature increases up to 4 °C upon the application of an alternating magnetic field. These magnetic microparticles were mixed with drug-loaded microparticles, and, subsequently, this mixture was embedded within a Pluronic thermoresponsive hydrogel that is capable of being in the gel state at 37 °C. The proposed DDS was capable of slowly releasing methylene blue, used as a model drug, for up to 9 days. The developed hydrogel/microparticle system had a smaller rate of drug release compared with microparticles alone. This system proved to be a potential thermoresponsive DDS suitable for magnetic hyperthermia applications, thus enabling a synergistic treatment for cancer.
Keywords: Pluronic; hydrogels; microparticles; rheology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Bosio V.E., Cacicedo M.L., Calvignac B., León I., Beuvier T., Boury F., Castro G.R. Synthesis and characterization of CaCO3 –biopolymer hybrid nanoporous microparticles for controlled release of doxorubicin. Colloids Surf. B Biointerfaces. 2014;123:158–169. doi: 10.1016/j.colsurfb.2014.09.011. - DOI - PubMed
-
- Balakrishnan B., Jayakrishnan A. Injectable Hydrogels for Biomedical Applications. In: Nair L.S., editor. Injectable Hydrogels for Regenerative Engineering. Imperial College Press; London, UK: 2016. pp. 33–94. - DOI
LinkOut - more resources
Full Text Sources

