Protective Effects of Sitagliptin on Streptozotocin-Induced Hepatic Injury in Diabetic Rats: A Possible Mechanisms
- PMID: 38131990
- PMCID: PMC10743245
- DOI: 10.3390/diseases11040184
Protective Effects of Sitagliptin on Streptozotocin-Induced Hepatic Injury in Diabetic Rats: A Possible Mechanisms
Abstract
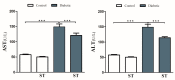
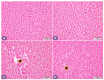
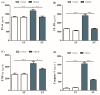
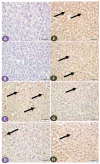
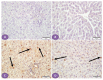
Diabetes is a ubiquitous disease that causes several complications. It is associated with insulin resistance, which affects the metabolism of proteins, carbohydrates, and fats and triggers liver diseases such as fatty liver disease, steatohepatitis, fibrosis, and cirrhosis. Despite the effectiveness of Sitagliptin (ST) as an antidiabetic drug, its role in diabetes-induced liver injury is yet to be fully investigated. Therefore, this study aims to investigate the effect of ST on hepatic oxidative injury, inflammation, apoptosis, and the mTOR/NF-κB/NLRP3 signaling pathway in streptozotocin (STZ)-induced liver injury. Rats were allocated into four groups: two nondiabetic groups, control rats and ST rats (100 mg/kg), and two diabetic groups induced by STZ, and they received either normal saline or ST for 90 days. Diabetic rats showed significant hyperglycemia, hyperlipidemia, and elevation in liver enzymes. After STZ induction, the results revealed remarkable increases in hepatic oxidative stress, inflammation, and hepatocyte degeneration. In addition, STZ upregulated the immunoreactivity of NF-κB/p65, NLRP3, and mTOR but downregulated IKB-α in liver tissue. The use of ST mitigated metabolic and hepatic changes induced by STZ; it also reduced oxidative stress, inflammation, and hepatocyte degeneration. The normal expression of NF-κB/p65, NLRP3, mTOR, and IKB-α were restored with ST treatment. Based on that, our study revealed for the first time the hepatoprotective effect of ST that is mediated by controlling inflammation, oxidative stress, and mTOR/NF-κB/NLRP3 signaling.
Keywords: STZ; diabetic; dipeptidyl peptidase-4 inhibitor; mTOR/NF-κB/NLRP3 signaling; sitagliptin.
Conflict of interest statement
The authors declare no potential conflict of interest related to the research, authorship, and/or publication of this article.
Figures



References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
-
- Jarrar M., Al-Bsheish M., Abusalah M.A.H., Albaker W., Alsyouf A., Al-Mugheed K., Issa M.R., Alumran A. Prevalence of type 2 diabetes mellitus in the general population of Saudi Arabia, 2000–2020: A systematic review and meta-analysis of observational studies. Saudi J. Med. Med. Sci. 2023;11:1. doi: 10.4103/sjmms.sjmms_394_22. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

