Use of the Therapy App Prescinde for Increasing Adherence to Smoking Cessation Treatment
- PMID: 38132011
- PMCID: PMC10742439
- DOI: 10.3390/healthcare11243121
Use of the Therapy App Prescinde for Increasing Adherence to Smoking Cessation Treatment
Abstract
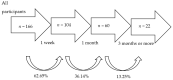
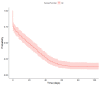
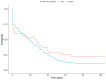
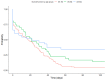
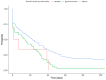
Tobacco use poses major health risks and is a major contributor to causes of death worldwide. Mobile phone-based cessation apps for this substance are gaining popularity, often used as a component of traditional interventions. This study aimed to analyze adherence to an intervention using a mobile phone application (App-therapy Prescinde (v1)) as a function of sociodemographic variables (age, gender, educational level, and profession) as well as the primary activities supported by the app (reducing tobacco or cannabis use and increasing physical exercise). The participants were recruited through the web pages of the Occupational Risk Prevention Service and the Psychology Clinic of the University of Granada during the COVID-19 confinement period. The application's contents include three components (self-report, motivational phrases, and goal setting). Our findings indicate that being male, being aged between 26 and 62, having a high school education, and being unemployed increase the likelihood of adherence to the Prescinde therapy app three months after usage. Our findings highlight the importance of developing new therapeutic approaches and conducting in-depth studies on the factors associated with adherence to tobacco cessation and cannabis cessation treatments via mobile phone applications.
Keywords: adherence; cannabis; mobile app; physical exercise; tobacco.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chu A., Chaiton M., Kaufman P., Goodwin R.D., Lin J., Hindocha C., Goodman S., Hammond D. Co-Use, Simultaneous Use, and Mixing of Cannabis and Tobacco: A Cross-National Comparison of Canada and the US by Cannabis Administration Type. Int. J. Environ. Res. Public Health. 2023;20:4206. doi: 10.3390/ijerph20054206. - DOI - PMC - PubMed
-
- International Agency for Research on Cancer World Health Organization. 2012. [(accessed on 1 November 2023)]. Available online: https://www3.paho.org/hq/index.php.
-
- Wallach J.D., Egilman A.C., Dhruva S.S., E McCarthy M., E Miller J., Woloshin S., Schwartz L.M., Ross J.S. Postmarket studies required by the US Food and Drug Administration for new drugs and biologics approved between 2009 and 2012: Cross sectional analysis. BMJ. 2018;361:k2031. doi: 10.1136/bmj.k2031. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

