Diagnostic Significance of Tryptase for Suspected Mast Cell Disorders
- PMID: 38132246
- PMCID: PMC10742504
- DOI: 10.3390/diagnostics13243662
Diagnostic Significance of Tryptase for Suspected Mast Cell Disorders
Abstract
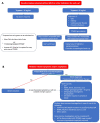
Tryptase has proven to be a very useful and specific marker to demonstrate mast cell activation and degranulation when an acute (i.e., within 4 h after the event) and baseline value (i.e., at least 24 h after the event) are compared and meet the consensus formula (i.e., an increase of 20% + 2). The upper limit of normal determined by the manufacturer is 11.4 ng/mL; however, this boundary has been the subject of debate. According to ECNM and AIM experts, the normal range of baseline tryptase should be 1 to 15 ng/mL. A genetic trait, hereditary alpha tryptasemia, characterized by an increased alpha coding TPSAB1 copy number is associated with a baseline value above 8 ng/mL. Elevated tryptase can also be found in chronic kidney disease, obesity, and hematological neoplasms. A tryptase > 20 ng/mL serves as a minor criterion to diagnose systemic mastocytosis and an increase in tryptase > 20% + 2 during an acute event is a required criterion in the diagnosis of mast cell activation syndrome. The goal of this review is to demonstrate the (in)significance of tryptase using some clinical vignettes and to provide a practical guide on how to manage and interpret an elevated tryptase level.
Keywords: HaT; MCAS; mastocytosis; tryptase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

