The Role of the Intestinal Microbiome in Multiple Sclerosis-Lessons to Be Learned from Hippocrates
- PMID: 38132289
- PMCID: PMC10740531
- DOI: 10.3390/biology12121463
The Role of the Intestinal Microbiome in Multiple Sclerosis-Lessons to Be Learned from Hippocrates
Abstract
Based on recent advances in research of chronic inflammatory conditions, there is a growing body of evidence that suggests a close correlation between the microbiota of the gastrointestinal tract and the physiologic activity of the immune system. This raises the idea that disturbances of the GI ecosystem contribute to the unfolding of chronic diseases including neurodegenerative pathologies. Here, we overview our current understanding on the putative interaction between the gut microbiota and the immune system from the aspect of multiple sclerosis, one of the autoimmune conditions accompanied by severe chronic neuroinflammation that affects millions of people worldwide.
Keywords: intestinal microbiome; macrophages; multiple sclerosis; regulatory T lymphocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
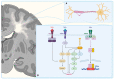


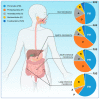

References
-
- Wylezinski L.S., Gray J.D., Polk J.B., Harmata A.J., Spurlock C.F., 3rd Illuminating an Invisible Epidemic: A Systemic Review of the Clinical and Economic Benefits of Early Diagnosis and Treatment in Inflammatory Disease and Related Syndromes. J. Clin. Med. 2019;8:493. doi: 10.3390/jcm8040493. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

