Interaction of Polyanionic and Polycationic Brushes with Globular Proteins and Protein-like Nanocolloids
- PMID: 38132536
- PMCID: PMC10741738
- DOI: 10.3390/biomimetics8080597
Interaction of Polyanionic and Polycationic Brushes with Globular Proteins and Protein-like Nanocolloids
Abstract
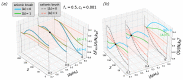
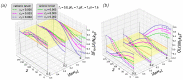
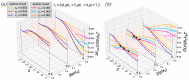
A large number of experimental studies have demonstrated that globular proteins can be absorbed from the solution by both polycationic and polyanionic brushes when the net charge of protein globules is of the same or of the opposite sign with respect to that of brush-forming polyelectrolyte chains. Here, we overview the results of experimental studies on interactions between globular proteins and polycationic or polyanionic brushes, and present a self-consistent field theoretical model that allows us to account for the asymmetry of interactions of protein-like nanocolloid particles comprising weak (pH-sensitive) cationic and anionic groups with a positively or negatively charged polyelectrolyte brush. The position-dependent insertion free energy and the net charge of the particle are calculated. The theoretical model predicts that if the numbers of cationic and anionic ionizable groups of the protein are approximately equal, then the interaction patterns for both cationic and anionic brushes at equal offset on the "wrong side" from the isoelectric point (IEP), i.e., when the particle and the brush charge are of the same sign, are similar. An essential asymmetry in interactions of particles with polycationic and polyanionic brushes is predicted when fractions of cationic and anionic groups differ significantly. That is, at a pH above IEP, the anionic brush better absorbs negatively charged particles with a larger fraction of ionizable cationic groups and vice versa.
Keywords: Poisson-Boltzmann theory; electrostatic interactions; ionic strength; polyelectrolyte brushes; protein absorption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dubin P., Bock J., Davis R., Schulz D.N., Thies C. Macromolecular Complexes in Chemistry and Biology. Springer; New York, NY, USA: 1994.
-
- Mattison K.W., Brittain I.J., Dubin P.L. Protein-polyelectrolyte phase boundaries. Biotechnol. Prog. 1995;11:632–637. doi: 10.1021/bp00036a005. - DOI
-
- Mattison K.W., Dubin P.L., Brittain I.J. Complex formation between bovine serum albumin and strong polyelectrolytes: Effect of polymer charge density. J. Phys. Chem. B. 1998;102:3830–3836. doi: 10.1021/jp980486u. - DOI
-
- Mattison K.W., Wang Y., Grymonpré K., Dubin P.L. Micro- and macro-phase behavior in protein-polyelectrolyte complexes. Macromol. Symp. 1999;140:53–76. doi: 10.1002/masy.19991400107. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

