HER2-Targeted Therapy-From Pathophysiology to Clinical Manifestation: A Narrative Review
- PMID: 38132657
- PMCID: PMC10743885
- DOI: 10.3390/jcdd10120489
HER2-Targeted Therapy-From Pathophysiology to Clinical Manifestation: A Narrative Review
Abstract
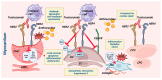
Trastuzumab is the primary treatment for all stages of HER2-overexpressing breast cancer in patients. Though discovered over 20 years ago, trastuzumab-induced cardiotoxicity (TIC) remains a research topic in cardio-oncology. This review explores the pathophysiological basis of TIC and its clinical manifestations. Their understanding is paramount for early detection and cardioprotective treatment. Trastuzumab renders cardiomyocytes susceptible by inhibiting the cardioprotective NRG-1/HER2/HER4 signaling pathway. The drug acts on HER2-receptor-expressing cardiomyocytes, endothelium, and cardiac progenitor cells (see the Graphical Abstract). The activation of immune cells, fibroblasts, inflammation, and neurohormonal systems all contribute to the evolution of TIC. A substantial amount of research demonstrates that trastuzumab induces overt and subclinical left ventricular (LV) systolic failure. Data suggest the development of right ventricular damage, LV diastolic dysfunction, and heart failure with preserved ejection fraction. Further research is needed to define a chronological sequence of cardiac impairments to guide the proper timing of cardioprotection implementation.
Keywords: cardiotoxicity; clinical manifestation; pathophysiology; trastuzumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Strauss E., Herceptin—A Targeted Antibody Therapy for Breast Cancer 2019 Lasker~DeBakey Clinical Medical Research Award. 2019. [(accessed on 28 October 2023)]. Available online: https://laskerfoundation.org/winners/herceptin-a-targeted-antibody-thera....
-
- Perez E.A., Romond E.H., Suman V.J., Jeong J.H., Sledge G., Geyer C.E., Jr., Martino S., Rastogi P., Gralow J., Swain S.M., et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. - DOI - PMC - PubMed
-
- Gennari A., André F., Barrios C.H., Cortés J., de Azambuja E., DeMichele A., Dent R., Fenlon D., Gligorov J., Hurvitz S.A., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. - DOI - PubMed
-
- Curigliano G., Castelo-Branco L., Gennari A., Harbeck N., Criscitiello C., Trapani D., on behalf of the Clinical Practice Guideline Author Group ESMO Metastatic Breast Cancer Living Guidelines, v1.1 May 2023. [(accessed on 28 October 2023)]. Available online: https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-liv...
-
- Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., Zackrisson S., Senkus E., ESMO Guidelines Committee Early Breast Cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

