Model-Based Design to Enhance Neotissue Formation in Additively Manufactured Calcium-Phosphate-Based Scaffolds
- PMID: 38132817
- PMCID: PMC10744304
- DOI: 10.3390/jfb14120563
Model-Based Design to Enhance Neotissue Formation in Additively Manufactured Calcium-Phosphate-Based Scaffolds
Abstract
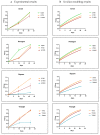
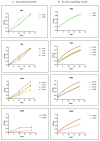
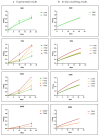
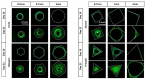
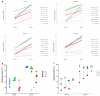
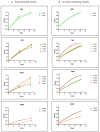
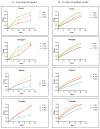
In biomaterial-based bone tissue engineering, optimizing scaffold structure and composition remains an active field of research. Additive manufacturing has enabled the production of custom designs in a variety of materials. This study aims to improve the design of calcium-phosphate-based additively manufactured scaffolds, the material of choice in oral bone regeneration, by using a combination of in silico and in vitro tools. Computer models are increasingly used to assist in design optimization by providing a rational way of merging different requirements into a single design. The starting point for this study was an in-house developed in silico model describing the in vitro formation of neotissue, i.e., cells and the extracellular matrix they produced. The level set method was applied to simulate the interface between the neotissue and the void space inside the scaffold pores. In order to calibrate the model, a custom disk-shaped scaffold was produced with prismatic canals of different geometries (circle, hexagon, square, triangle) and inner diameters (0.5 mm, 0.7 mm, 1 mm, 2 mm). The disks were produced with three biomaterials (hydroxyapatite, tricalcium phosphate, and a blend of both). After seeding with skeletal progenitor cells and a cell culture for up to 21 days, the extent of neotissue growth in the disks' canals was analyzed using fluorescence microscopy. The results clearly demonstrated that in the presence of calcium-phosphate-based materials, the curvature-based growth principle was maintained. Bayesian optimization was used to determine the model parameters for the different biomaterials used. Subsequently, the calibrated model was used to predict neotissue growth in a 3D gyroid structure. The predicted results were in line with the experimentally obtained ones, demonstrating the potential of the calibrated model to be used as a tool in the design and optimization of 3D-printed calcium-phosphate-based biomaterials for bone regeneration.
Keywords: 3D printing; biomaterials; bone tissue engineering; computer modeling and simulation; dental bone regeneration; in silico medicine; optimal design; porosity; porous scaffold.
Conflict of interest statement
The authors declare no conflict of interest. Justine Pirson is an employee of Wishone SA. The paper reflects the views of the scientists, and not the company. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Bone|Definition, Anatomy, & Composition|Britannica. [(accessed on 30 October 2023)]. Available online: https://www.britannica.com/science/bone-anatomy.
Grants and funding
LinkOut - more resources
Full Text Sources

