Tetraenone A: A New β-Ionone Derivative from Tetraena aegyptia
- PMID: 38132884
- PMCID: PMC10744760
- DOI: 10.3390/metabo13121202
Tetraenone A: A New β-Ionone Derivative from Tetraena aegyptia
Abstract
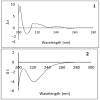
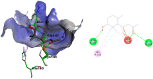
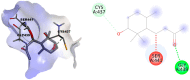
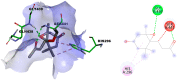
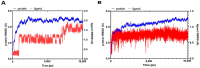
In this study, the chemical investigation of Tetraena aegyptia (Zygophyllaceae) led to the identification of a new megastigmene derivative, tetraenone A ((2S, 5R, 6R, 7E)-2-hydroxy-5,6-dihydro-β-ionone) (1), along with (3S, 5R, 6S, 7E)-3-hydroxy-5,6-epoxy-5,6-dihydro-β-ionone- (2), 3,4-dihydroxy-cinnamyl alcohol-4-glucoside (3), 3β,19α-dihydroxy-ursan-28-oic acid (4), quinovic acid (5), p-coumaric acid (6), and ferulic acid (7), for the first time. The chemical structures of 1-7 were confirmed by analysis of their 1D and 2D NMR and HRESIMS spectra and by their comparison with the relevant literature. The absolute configurations of 1 and 2 were assigned based on NOESY interactions and ECD spectra. Conformational analysis showed that 1 existed exclusively in one of the two theoretically possible chair conformers with a predominant s-trans configuration for the 3-oxobut-1-en-1-yl group with the ring, while the half-chair conformer had a pseudo-axial hydroxy group that was predominant over the other half-chair conformation. Boat conformations were not among the most stable conformations, and the s-trans isomerism was in favor of s-cis configuration. In silico investigation revealed that 1 and 2 had more favorable binding interactions with Mpro rather than with TMPRSS2. Accordingly, molecular dynamic simulations were performed on the complexes of compounds 1 and 2 with Mpro to explore the stability of their interaction with the target protein structure. Compounds 1 and 2 might offer a possible starting point for developing covalent inhibitors of Mpro of SARS-CoV-2.
Keywords: SARS-CoV-2; Tetraena aegyptia; drug discovery; health and wellbeing; life on land; megastigmene; tetraenone A.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Shawky E.M., Gabr N.M., El-Gindi M.R., Mekky R.H. A Comprehensive Review on Genus Zygophyllum. J. Adv. Pharm. Res. 2019;3:1–16. doi: 10.21608/aprh.2019.5699.1066. - DOI
-
- Belguidoum M., Dendougui H., Kendour Z., Belfar A., Bensaci C., Hadjadj M. Antioxidant activities, phenolic, flavonoid and tannin contents of endemic Zygophyllum Cornutum Coss. From Algerian Sahara. Der Pharma Chem. 2015;7:312–317.
-
- Guenzet A., Krouf D., Zennaki S., Berzou S. Zygophyllum gaetulum Aqueous Extract Protects against Diabetic Dyslipidemia and Attenuates Liver and Kidney Oxidative Damage in Streptozotocin Induced-Diabetic Rats. Int. J. Pharm. Sci. Res. 2014;5:4709.
-
- Kchaou M., Ben Salah H.B., Mhiri R., Allouche N. Anti-oxidant and anti-acetylcholinesterase activities of Zygophyllum album. Bangladesh J. Pharmacol. 2016;11:54–62. doi: 10.3329/bjp.v11i1.25463. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

