Marine-Fungi-Derived Gliotoxin Promotes Autophagy to Suppress Mycobacteria tuberculosis Infection in Macrophage
- PMID: 38132937
- PMCID: PMC10745037
- DOI: 10.3390/md21120616
Marine-Fungi-Derived Gliotoxin Promotes Autophagy to Suppress Mycobacteria tuberculosis Infection in Macrophage
Abstract
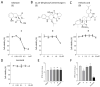
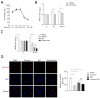
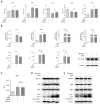
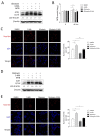
The Mycobacterium tuberculosis (MTB) infection causes tuberculosis (TB) and has been a long-standing public-health threat. It is urgent that we discover novel antitubercular agents to manage the increased incidence of multidrug-resistant (MDR) or extensively drug-resistant (XDR) strains of MTB and tackle the adverse effects of the first- and second-line antitubercular drugs. We previously found that gliotoxin (1), 12, 13-dihydroxy-fumitremorgin C (2), and helvolic acid (3) from the cultures of a deep-sea-derived fungus, Aspergillus sp. SCSIO Ind09F01, showed direct anti-TB effects. As macrophages represent the first line of the host defense system against a mycobacteria infection, here we showed that the gliotoxin exerted potent anti-tuberculosis effects in human THP-1-derived macrophages and mouse-macrophage-leukemia cell line RAW 264.7, using CFU assay and laser confocal scanning microscope analysis. Mechanistically, gliotoxin apparently increased the ratio of LC3-II/LC3-I and Atg5 expression, but did not influence macrophage polarization, IL-1β, TNF-a, IL-10 production upon MTB infection, or ROS generation. Further study revealed that 3-MA could suppress gliotoxin-promoted autophagy and restore gliotoxin-inhibited MTB infection, indicating that gliotoxin-inhibited MTB infection can be treated through autophagy in macrophages. Therefore, we propose that marine fungi-derived gliotoxin holds the promise for the development of novel drugs for TB therapy.
Keywords: Mycobacterium tuberculosis (MTB); autophagy; gliotoxin; macrophages; marine natural product.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Antituberculosis compounds from a deep-sea-derived fungus Aspergillus sp. SCSIO Ind09F01.Nat Prod Res. 2017 Aug;31(16):1958-1962. doi: 10.1080/14786419.2016.1266353. Epub 2017 Jan 9. Nat Prod Res. 2017. PMID: 28068839
-
Pomalidomide promotes macrophage control of Mycobacterium tuberculosis via enhancing HDAC6-mediated autophagy.Int Immunopharmacol. 2025 Jun 17;158:114831. doi: 10.1016/j.intimp.2025.114831. Epub 2025 May 14. Int Immunopharmacol. 2025. PMID: 40373598
-
The Activities and Secretion of Cytokines Caused by Delamanid on Macrophages Infected by Multidrug-Resistant Mycobacterium tuberculosis Strains.Front Immunol. 2021 Dec 24;12:796677. doi: 10.3389/fimmu.2021.796677. eCollection 2021. Front Immunol. 2021. PMID: 35003120 Free PMC article.
-
Nanomaterial-mediated host directed therapy of tuberculosis by manipulating macrophage autophagy.J Nanobiotechnology. 2024 Oct 8;22(1):608. doi: 10.1186/s12951-024-02875-w. J Nanobiotechnology. 2024. PMID: 39379986 Free PMC article. Review.
-
The Macrophage Response to Mycobacterium tuberculosis and Opportunities for Autophagy Inducing Nanomedicines for Tuberculosis Therapy.Front Cell Infect Microbiol. 2021 Feb 8;10:618414. doi: 10.3389/fcimb.2020.618414. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33628745 Free PMC article. Review.
Cited by
-
Marine-Derived Leads as Anticancer Candidates by Disrupting Hypoxic Signaling through Hypoxia-Inducible Factors Inhibition.Mar Drugs. 2024 Mar 23;22(4):143. doi: 10.3390/md22040143. Mar Drugs. 2024. PMID: 38667760 Free PMC article. Review.
-
The Antitubercular Activities of Natural Products with Fused-Nitrogen-Containing Heterocycles.Pharmaceuticals (Basel). 2024 Feb 6;17(2):211. doi: 10.3390/ph17020211. Pharmaceuticals (Basel). 2024. PMID: 38399426 Free PMC article. Review.
References
-
- Hameed H.M.A., Islam M.M., Chhotaray C., Wang C., Liu Y., Tan Y.J., Li X.J., Tan S.Y., Delorme V., Yew W.W., et al. Molecular targets related drug resistance mechanisms in MDR-, XDR-, and TDR-Mycobacterium tuberculosis strains. Front. Cell Infect. Microbiol. 2018;8:114. doi: 10.3389/fcimb.2018.00114. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- GDNRC[2022]35, GDOE[2019]A28/the Special Funds for Promoting Economic Development (Marine Economic Development) of Guangdong Province
- 2021A1515011711/Natural Science Foundation of Guangdong Province
- Yonghong Liu/the Special Fund for Bagui Scholars of Guangxi
- 2022C038/the Scientific Research Foundation of Guangxi University of Chinese Medicine
LinkOut - more resources
Full Text Sources
Medical
Research Materials

