Polar Lipids of Marine Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis Mitigate the LPS-Induced Pro-Inflammatory Response in Macrophages
- PMID: 38132950
- PMCID: PMC10745121
- DOI: 10.3390/md21120629
Polar Lipids of Marine Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis Mitigate the LPS-Induced Pro-Inflammatory Response in Macrophages
Abstract
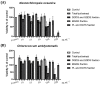
Microalgae are recognized as a relevant source of bioactive compounds. Among these bioactive products, lipids, mainly glycolipids, have been shown to present immunomodulatory properties with the potential to mitigate chronic inflammation. This study aimed to evaluate the anti-inflammatory effect of polar lipids isolated from Nannochloropsis oceanica and Chlorococcum amblystomatis. Three fractions enriched in (1) digalactosyldiacylglycerol (DGDG) and sulfoquinovosyldiacylglycerol (SQDG), (2) monogalactosyldiacylglycerol (MGDG), and (3) diacylglyceryl-trimethylhomoserine (DGTS) and phospholipids (PL) were obtained from the total lipid extracts (TE) of N. oceanica and C. amblystomatis, and their anti-inflammatory effect was assessed by analyzing their capacity to counteract nitric oxide (NO) production and transcription of pro-inflammatory genes Nos2, Ptgs2, Tnfa, and Il1b in lipopolysaccharide (LPS)-activated macrophages. For both microalgae, TE and Fractions 1 and 3 strongly inhibited NO production, although to different extents. A strong reduction in the LPS-induced transcription of Nos2, Ptgs2, Tnfa, and Il1b was observed for N. oceanica and C. amblystomatis lipids. The most active fractions were the DGTS-and-PL-enriched fraction from N. oceanica and the DGDG-and-SQDG-enriched fraction from C. amblystomatis. Our results reveal that microalgae lipids have strong anti-inflammatory capacity and may be explored as functional ingredients or nutraceuticals, offering a natural solution to tackle chronic inflammation-associated diseases.
Keywords: Chlorococcum amblystomatis; Nannochloropsis oceanica; anti-inflammatory activity; betaine lipids; glycolipids; lipidomics; lipids; microalgae; phospholipids.
Conflict of interest statement
The authors declare that author from Allmicroalgae—Natural Products S.A. did not have any role in designing and conducting of the study and analyzing the data.
Figures






References
-
- Malhotra S., Singh A.P. Algae, Traditional Medicine, and Pharmacological Advances. Int. J. Algae. 2008;10:299–308. doi: 10.1615/InterJAlgae.v10.i3.80. - DOI
-
- Silva M., Kamberovic F., Uota S.T., Kovan I.-M., Viegas C.S.B., Simes D.C., Gangadhar K.N., Varela J., Barreira L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022;12:5877. doi: 10.3390/app12125877. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

