Combination Therapy with Enalapril and Paricalcitol Ameliorates Streptozotocin Diabetes-Induced Testicular Dysfunction in Rats via Mitigation of Inflammation, Apoptosis, and Oxidative Stress
- PMID: 38133142
- PMCID: PMC10747062
- DOI: 10.3390/pathophysiology30040041
Combination Therapy with Enalapril and Paricalcitol Ameliorates Streptozotocin Diabetes-Induced Testicular Dysfunction in Rats via Mitigation of Inflammation, Apoptosis, and Oxidative Stress
Abstract
Background: As the impacts of diabetes-induced reproductive damage are now evident in young people, we are now in urgent need to devise new ways to protect and enhance the reproductive health of diabetic people. The present study aimed to evaluate the protective effects of enalapril (an ACE inhibitor) and paricalcitol (a vitamin D analog), individually or in combination, on streptozotocin (STZ)-diabetes-induced testicular dysfunction in rats and to identify the possible mechanisms for this protection.
Material and methods: This study was carried out on 50 male Sprague-Dawley rats; 10 normal rats were allocated as a non-diabetic control group. A total of 40 rats developed diabetes after receiving a single dose of STZ; then, the diabetic rats were divided into four groups of equivalent numbers assigned as diabetic control, enalapril-treated, paricalcitol-treated, and combined enalapril-and-paricalcitol-treated groups. The effects of mono and combined therapy with paricalcitol and enalapril on testicular functions, sperm activity, glycemic state oxidative stress, and inflammatory parameters, as well as histopathological examinations, were assessed in comparison with the normal and diabetic control rats.
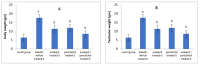
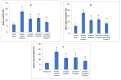
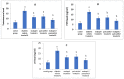
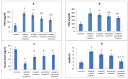
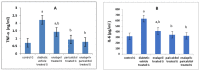
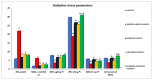
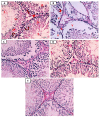
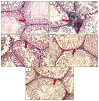
Results: As a result of diabetes induction, epididymal sperm count, sperm motility, serum levels of testosterone, follicle-stimulating hormone (FSH) as well as luteinizing hormone (LH), and the antioxidant enzyme activities, were significantly decreased, while abnormal sperm (%), insulin resistance, nitric oxide (NO), malondialdehyde (MDA), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were significantly increased, along with severe distortion of the testicular structure. Interestingly, treatment with paricalcitol and enalapril, either alone or in combination, significantly improved the sperm parameters, increased antioxidant enzyme activities in addition to serum levels of testosterone, FSH, and LH, reduced insulin resistance, IL-6, and TNF-α levels, and finally ameliorated the diabetes-induced testicular oxidative stress and histopathological damage, with somewhat superior effect for paricalcitol monotherapy and combined therapy with both drugs compared to monotherapy with enalapril alone.
Conclusions: Monotherapy with paricalcitol and its combination therapy with enalapril has a somewhat superior effect in improving diabetes-induced testicular dysfunction (most probably as a result of their hypoglycemic, antioxidant, anti-inflammatory, and anti-apoptotic properties) compared with monotherapy with enalapril alone in male rats, recommending a synergistic impact of both drugs.
Keywords: diabetes; enalapril; oxidative stress; paricalcitol; testicular dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
