Onabotulinumtoxin-A: Previous Prophylactic Treatment Might Improve Subsequent Anti-CGRP Monoclonal Antibodies Response in Patients with Chronic Migraine
- PMID: 38133181
- PMCID: PMC10747467
- DOI: 10.3390/toxins15120677
Onabotulinumtoxin-A: Previous Prophylactic Treatment Might Improve Subsequent Anti-CGRP Monoclonal Antibodies Response in Patients with Chronic Migraine
Abstract
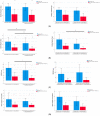
The aim of the present study was to evaluate whether previous preventive treatment with onabotulinumtoxin-A might influence subsequent clinical response following a switch to anti-CGRP monoclonal antibodies (mAbs). The present retrospective study was conducted at the Headache Centre-Neurology Clinic at the Spedali Civili Hospital of Brescia between November 2018 and May 2023. The primary objective was to assess clinical outcome (monthly headache days (MHDs), monthly migraine days (MMDs), mean analgesics consumption, and clinical disability according to Migraine Disability Assessment (MIDAS)) following three months (T3) of preventive treatment with anti-CGRP mAbs comparing patients who did and those who did not previously receive treatment with Onabotulinumtoxin-A. Moreover, we aimed to evaluate whether the clinical response to anti-CGRP mAbs was affected by the number of previous Onabotulinumtoxin-A administrations. At T3, compared to Onabotulinumtoxin-A naïve patients, patients who previously received Onabotulinumtoxin-A documented fewer MMDs (3.3 ± 3.7 versus 5.2 ± 5.0; p = 0.017) and a lower MIDAS score (23.2 ± 20.9 versus 37.4 ± 39.6; p = 0.013). Patients who received at least 3 onabotulinumtoxin-A administrations documented, at T3, lower MMDs compared to those who received fewer cycles (respectively, 2.1 ± 2.7 vs. 6.5 ± 4.4; p = 0.024). In conclusion, according to our data, previous treatment with onabotulinumtoxin-A might improve subsequent response to anti-CGRP mAbs preventive treatment.
Keywords: CGRP; anti-CGRP monoclonal antibodies; chronic migraine; migraine; onabotulinumtoxin A; prevention.
Conflict of interest statement
Francesca Schiano di Cola has received speaker honoraria from Eli-Lilly, Lundbeck, and Novartis. Renata Rao has received speaker honoraria from Eli-Lilly, Novartis, Lundbeck, and Teva. Alessandro Padovani is a consultant, has served on the scientific advisory board of GE Healthcare, Eli-Lilly, and Actelion Ltd. Pharmaceuticals, and has received speaker honoraria from Nutricia, PIAM, Langstone Technology, GE Healthcare, Lilly, Lundbeck, UCB Pharma, and Chiesi Pharmaceuticals. He is funded by a grant from the Ministry of the University (MURST).
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

