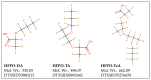
Dose Response, Dosimetric, and Metabolic Evaluations of Replacement PFAS Perfluoro-(2,5,8-trimethyl-3,6,9-trioxadodecanoic) Acid (HFPO-TeA)
- PMID: 38133352
- PMCID: PMC10747602
- DOI: 10.3390/toxics11120951
Dose Response, Dosimetric, and Metabolic Evaluations of Replacement PFAS Perfluoro-(2,5,8-trimethyl-3,6,9-trioxadodecanoic) Acid (HFPO-TeA)
Abstract
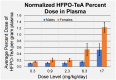
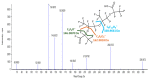
Few studies are available on the environmental and toxicological effects of perfluoroalkyl ether carboxylic acids (PFECAs), such as GenX, which are replacing legacy PFAS in manufacturing processes. To collect initial data on the toxicity and toxicokinetics of a longer-chain PFECA, male and female Sprague Dawley rats were exposed to perfluoro-(2,5,8-trimethyl-3,6,9-trioxadodecanoic) acid (HFPO-TeA) by oral gavage for five days over multiple dose levels (0.3-335.2 mg/kg/day). Clinically, we observed mortality at doses >17 mg/kg/day and body weight changes at doses ≤17 mg/kg/day. For the 17 mg/kg/day dose level, T3 and T4 thyroid hormone concentrations were significantly decreased (p < 0.05) from controls and HFPO-TeA plasma concentrations were significantly different between sexes. Non-targeted analysis of plasma and in vitro hepatocyte assay extractions revealed the presence of another GenX oligomer, perfluoro-(2,5-dimethyl-3,6-dioxanonanoic) acid (HFPO-TA). In vitro to in vivo extrapolation (IVIVE) parameterized with in vitro toxicokinetic data predicted steady-state blood concentrations that were within seven-fold of those observed in the in vivo study, demonstrating reasonable predictivity. The evidence of thyroid hormone dysregulation, sex-based differences in clinical results and dosimetry, and IVIVE predictions presented here suggest that the replacement PFECA HFPO-TeA induces a complex and toxic exposure response in rodents.
Keywords: IVIVE; PFAS; PFECA; dosimetry; hepatic clearance; non-targeted analysis (NTA); plasma protein binding; thyroid disruption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Maternal and Neonatal Effects of Maternal Oral Exposure to Perfluoro-2-methoxyacetic Acid (PFMOAA) during Pregnancy and Early Lactation in the Sprague-Dawley Rat.Environ Sci Technol. 2024 Jan 16;58(2):1064-1075. doi: 10.1021/acs.est.3c08559. Epub 2024 Jan 1. Environ Sci Technol. 2024. PMID: 38163761 Free PMC article.
-
First Report on the Bioaccumulation and Trophic Transfer of Perfluoroalkyl Ether Carboxylic Acids in Estuarine Food Web.Environ Sci Technol. 2022 May 17;56(10):6046-6055. doi: 10.1021/acs.est.1c00965. Epub 2021 Jul 23. Environ Sci Technol. 2022. PMID: 34296857
-
Accumulation and glucocorticoid signaling suppression by four emerging perfluoroethercarboxylic acids based on animal exposure and cell testing.Environ Int. 2023 Aug;178:108092. doi: 10.1016/j.envint.2023.108092. Epub 2023 Jul 13. Environ Int. 2023. PMID: 37463541
-
Comparative analysis of the physicochemical, toxicokinetic, and toxicological properties of ether-PFAS.Toxicol Appl Pharmacol. 2021 Jul 1;422:115531. doi: 10.1016/j.taap.2021.115531. Epub 2021 Apr 29. Toxicol Appl Pharmacol. 2021. PMID: 33933458 Review.
-
Legacy and Emerging Per- and Polyfluoroalkyl Substances: Analytical Techniques, Environmental Fate, and Health Effects.Int J Mol Sci. 2021 Jan 20;22(3):995. doi: 10.3390/ijms22030995. Int J Mol Sci. 2021. PMID: 33498193 Free PMC article. Review.
Cited by
-
Closing the Gaps in Understanding PFAS Toxicology and Metabolism.Toxics. 2024 Dec 27;13(1):19. doi: 10.3390/toxics13010019. Toxics. 2024. PMID: 39853019 Free PMC article.
-
The pollutant perfluorohexane sulfonate (PFHxS) reduces serum thyroxine but does not alter thyroid action in the postnatal rat brain.Environ Int. 2024 Aug;190:108838. doi: 10.1016/j.envint.2024.108838. Epub 2024 Jun 19. Environ Int. 2024. PMID: 38963985 Free PMC article.
-
Updated Guidance for Communicating PFAS Identification Confidence with Ion Mobility Spectrometry.bioRxiv [Preprint]. 2025 May 17:2025.01.27.634925. doi: 10.1101/2025.01.27.634925. bioRxiv. 2025. Update in: Environ Sci Technol. 2025 Aug 26;59(33):17711-17721. doi: 10.1021/acs.est.5c01354. PMID: 39975284 Free PMC article. Updated. Preprint.
-
In Vitro Hepatic Clearance Evaluations of Per- and Polyfluoroalkyl Substances (PFAS) across Multiple Structural Categories.Toxics. 2024 Sep 14;12(9):672. doi: 10.3390/toxics12090672. Toxics. 2024. PMID: 39330600 Free PMC article.
-
Non-Targeted Analysis (NTA) of Plasma and Liver from Sprague Dawley Rats Exposed to Perfluorohexanesulfonamide (PFHxSA), a Precursor to Perfluorohexane Sulfonic Acid (PFHxS).Toxics. 2025 Jun 21;13(7):523. doi: 10.3390/toxics13070523. Toxics. 2025. PMID: 40710967 Free PMC article.
References
-
- OCED/UNEP . Synthesis Paper of per and Poly Fluorinated Chemicals (PFC) Organisation for Economic Cooperation and Development and United Nations Environmental Program Global PFC Group; Paris, France: 2013.
-
- Kurwadkar S., Dane J., Kanel S.R., Nadagouda M.N., Cawdrey R.W., Ambade B., Struckhoff G.C., Wilkin R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022;809:151003. doi: 10.1016/j.scitotenv.2021.151003. - DOI - PMC - PubMed
-
- Bolan N., Sarkar B., Vithanage M., Singh G., Tsang D.C.W., Mukhopadhyay R., Ramadass K., Vinu A., Sun Y., Ramanayaka S., et al. Distribution, behavior, bioavailability and remediation of poly- and per-fluoroalky substances (PFAS) in solid biowastes and biowaste-treated soil. Environ. Int. 2021;155:106600. doi: 10.1016/j.envint.2021.106600. - DOI - PubMed
LinkOut - more resources
Full Text Sources

