Assessment of Biofilm Growth on Microplastics in Freshwaters Using a Passive Flow-Through System
- PMID: 38133388
- PMCID: PMC10748376
- DOI: 10.3390/toxics11120987
Assessment of Biofilm Growth on Microplastics in Freshwaters Using a Passive Flow-Through System
Abstract
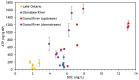
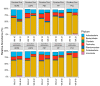
Biofilms that colonize on the surface of microplastics (MPs) in freshwaters may pose a potential health risk. This study examined factors that influence MP-associated biofilm growth, including polymer type, degree of weathering, and source water quality. Weathered MPs produced in-lab were employed in biofilm trials conducted on site using a passive flow-through system with raw water at drinking water treatment facility intakes. Adenosine triphosphate (ATP) was used to quantify biofilm abundance; biofilm composition was assessed via metagenomic sequencing. Biofilm growth was observed on all polymer types examined and most prevalent on polyvinyl chloride (PVC), where ATP levels were 6 to 12 times higher when compared to other polymers. Pathogen-containing species including Salmonella enterica and Escherichia coli were present on all polymers with relative abundance up to 13.7%. S. enterica was selectively enriched on weathered MPs in specific water matrices. These findings support the need to research the potential accumulation of pathogenic organisms on microplastic surfaces.
Keywords: ATP; HDPE; LDPE; PET; PP; PVC; biofilm; freshwater; metagenomics; weathering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- The California Water Board . Adoption of Definition of “Microplastics in Drinking Water”. The California Water Board; Sacramento, CA, USA: 2020.
-
- Triebskorn R., Braunbeck T., Grummt T., Hanslik L., Huppertsberg S., Jekel M., Knepper T.P., Krais S., Müller Y.K., Pittroff M., et al. Relevance of Nano- and Microplastics for Freshwater Ecosystems: A Critical Review. TrAC Trends Anal. Chem. 2019;110:375–392. doi: 10.1016/j.trac.2018.11.023. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

