Obicetrapib: Reversing the Tide of CETP Inhibitor Disappointments
- PMID: 38133847
- PMCID: PMC10838241
- DOI: 10.1007/s11883-023-01184-1
Obicetrapib: Reversing the Tide of CETP Inhibitor Disappointments
Abstract
Purpose of review: To discuss the history of cardiovascular outcomes trials of cholesteryl ester transfer protein (CETP) inhibitors and to describe obicetrapib, a next-generation, oral, once-daily, low-dose CETP inhibitor in late-stage development for dyslipidemia and atherosclerotic cardiovascular disease (ASCVD).
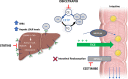
Recent findings: Phase 1 and 2 trials have evaluated the safety and lipid/lipoprotein effects of obicetrapib as monotherapy, in conjunction with statins, on top of high-intensity statins (HIS), and with ezetimibe on top of HIS. In ROSE2, 10 mg obicetrapib monotherapy and combined with 10 mg ezetimibe, each on top of HIS, significantly reduced low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), apolipoprotein B, total LDL particles, small LDL particles, small, dense LDL-C, and lipoprotein (a), and increased HDL-C. Phase 3 pivotal registration trials including a cardiovascular outcomes trial are underway. Obicetrapib has an excellent safety and tolerability profile and robustly lowers atherogenic lipoproteins and raises HDL-C. As such, obicetrapib may be a promising agent for the treatment of ASCVD.
Keywords: Atherosclerotic cardiovascular disease (ASCVD); Cardiovascular outcomes trial; Cholesteryl ester transfer protein (CETP) inhibitor; High-density lipoprotein cholesterol (HDL-C); Low-density lipoprotein cholesterol (LDL-C); Obicetrapib.
© 2023. The Author(s).
Conflict of interest statement
J.J.P.K, A.H., M.D., and M.H.D. are employees of NewAmsterdam Pharma and they also report that they receive stock or stock options. J.J.P.K is the Chief Scientific Officer of NewAmsterdam Pharma. A.H. is the Executive Director, R&D, NewAmsterdam Pharma. M.D. is the Chief Development Officer of NewAmsterdam Pharma. M.H.D. is the Chief Executive Officer of NewAmsterdam Pharma. M.R.D., as an employee of Midwest Biomedical Research, is a consultant for NewAmsterdam Pharma. In addition, M.R.D., as an employee of Midwest Biomedical Research, reports the following companies contracted consulting and/or research with their institution and it received the consulting fees and grant funding from Acasti Pharma, Beren Therapeutics, Eli Lilly and Company, Indiana University and Foundation, Matinas BioPharma, NewAmsterdam Pharma, and 89Bio.
Figures
References
-
- Kurasawa T, Yokoyama S, Miyake Y, Yamamura T, Yamamoto A. Rate of cholesteryl ester transfer between high and low density lipoproteins in human serum and a case with decreased transfer rate in association with hyperalphalipoproteinemia. J Biochem. 1985;98:1499–1508. doi: 10.1093/oxfordjournals.jbchem.a135418. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

