Taxonomic profiling of Nasutitermes takasagoensis microbiota to investigate the role of termites as vectors of bacteria linked to ironwood tree decline in Guam
- PMID: 38134025
- PMCID: PMC10745211
- DOI: 10.1371/journal.pone.0296081
Taxonomic profiling of Nasutitermes takasagoensis microbiota to investigate the role of termites as vectors of bacteria linked to ironwood tree decline in Guam
Abstract
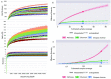
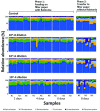
The ironwood tree (Casuarina equisetifolia, family Casuarinaceae), an indigenous agroforestry species in Guam, has been threatened by ironwood tree decline (IWTD) since 2002. Formation of bacterial ooze by the wilt pathogen from the Ralstonia solanacearum species complex and wetwood bacteria (primarily Klebsiella species) has been linked to IWTD. In addition, termite infestation of trees was statistically associated with IWTD. Termites are known carriers of a diverse microbiome. Therefore, we hypothesized that termites could be vectors of bacteria linked to IWTD. To investigate the potential role of termites as pathogen vectors, we employed next-generation 16S rRNA gene sequencing to describe the bacteria diversity of Nasutitermes takasagoensis (Family Termitidae) workers collected from 42 ironwood trees of different disease stages in Guam in association with tree-, plot-, and location-related factors. Nasutitermes takasagoensis workers account for the majority of termite infestations of ironwood trees. The bacterial phyla composition of N. takasagoensis workers was typical for wood-feeding higher termites consisting mainly of Spirochaetes and Fibrobacteres. However, Ralstonia species were not detected and Klebsiella species were rare even in termites collected from trees infected with Ralstonia and wetwood bacteria. Feeding experiments suggested that termites prefer to consume wood with low pathogen content over wood with high pathogen load. Termites were able to ingest Ralstonia but Ralstonia could not establish itself in healthy termite bodies. We concluded that N. takasagoensis workers are not vectors for Ralstonia spp. or the bacterial endophytes associated with wetwood (Klebsiella, Pantoea, Enterobacter, Citrobacter, and Erwinia) that were previously observed in IWTD-infested trees. The bacterial diversity in termite samples was significantly influenced by various factors, including Tree Health, Site Management, Plot Average Decline Severity, Proportion of Dead Trees in the Plot, Proportion of Trees with Termite Damage in the Plot, Presence of Ralstonia, and Altitude.
Copyright: © 2023 Setia et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Mersha Z, Schlub RL, Moore A. The state of ironwood (Casuarina equisetifolia subsp. equisetifolia) decline on the Pacific island of Guam. Phytopathology. 2009;99:S85.
-
- Schlub RL. Gago, Guam ironwood tree, Casuarina equisetifolia: past, present, future. Guam Cooperative Extension Service Publication. 2013. Available at: http://cnas-re.uog.edu/wp-content/uploads/2016/01/finalreducedwsareironw....
-
- Elevitch CR, Wilkinson KM. Agroforestry guides for Pacific Islands. vii. 2000. https://doi-org.libezp.lib.lsu.edu/https://www.agroforestry.net/afg/book...
-
- Athens JS, Ard JVW. Holocene vegetation, savanna origins and human settlement of Guam. In: A Pacific Odyssey: Archaeology and Anthropology in the Western Pacific. Papers in Honour of Jim Specht. Records of The Australian Museum, Supplement. 2004;29:15–30. doi: 10.3853/j.0812-7387.29.2004.1398 - DOI
-
- Pinyopusarerk K, House APN. Casuarina: an annotated bibliography of C. equisetifolia, C. junghuhniana and C. oligodon. ICRAF. 1993.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

