Method to determine whether sleep phenotypes are driven by endogenous circadian rhythms or environmental light by combining longitudinal data and personalised mathematical models
- PMID: 38134229
- PMCID: PMC10817199
- DOI: 10.1371/journal.pcbi.1011743
Method to determine whether sleep phenotypes are driven by endogenous circadian rhythms or environmental light by combining longitudinal data and personalised mathematical models
Abstract
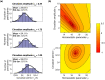
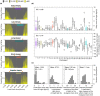
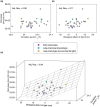
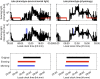
Sleep timing varies between individuals and can be altered in mental and physical health conditions. Sleep and circadian sleep phenotypes, including circadian rhythm sleep-wake disorders, may be driven by endogenous physiological processes, exogeneous environmental light exposure along with social constraints and behavioural factors. Identifying the relative contributions of these driving factors to different phenotypes is essential for the design of personalised interventions. The timing of the human sleep-wake cycle has been modelled as an interaction of a relaxation oscillator (the sleep homeostat), a stable limit cycle oscillator with a near 24-hour period (the circadian process), man-made light exposure and the natural light-dark cycle generated by the Earth's rotation. However, these models have rarely been used to quantitatively describe sleep at the individual level. Here, we present a new Homeostatic-Circadian-Light model (HCL) which is simpler, more transparent and more computationally efficient than other available models and is designed to run using longitudinal sleep and light exposure data from wearable sensors. We carry out a systematic sensitivity analysis for all model parameters and discuss parameter identifiability. We demonstrate that individual sleep phenotypes in each of 34 older participants (65-83y) can be described by feeding individual participant light exposure patterns into the model and fitting two parameters that capture individual average sleep duration and timing. The fitted parameters describe endogenous drivers of sleep phenotypes. We then quantify exogenous drivers using a novel metric which encodes the circadian phase dependence of the response to light. Combining endogenous and exogeneous drivers better explains individual mean mid-sleep (adjusted R-squared 0.64) than either driver on its own (adjusted R-squared 0.08 and 0.17 respectively). Critically, our model and analysis highlights that different people exhibiting the same sleep phenotype may have different driving factors and opens the door to personalised interventions to regularize sleep-wake timing that are readily implementable with current digital health technology.
Copyright: © 2023 Skeldon et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: DJD and ACS are consultants for F. Hoffmann-La Roche, Ltd.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources

