Flortaucipir tau PET findings from former professional and college American football players in the DIAGNOSE CTE research project
- PMID: 38134231
- PMCID: PMC10984430
- DOI: 10.1002/alz.13602
Flortaucipir tau PET findings from former professional and college American football players in the DIAGNOSE CTE research project
Abstract
Introduction: Tau is a key pathology in chronic traumatic encephalopathy (CTE). Here, we report our findings in tau positron emission tomography (PET) measurements from the DIAGNOSE CTE Research Project.
Method: We compare flortaucipir PET measures from 104 former professional players (PRO), 58 former college football players (COL), and 56 same-age men without exposure to repetitive head impacts (RHI) or traumatic brain injury (unexposed [UE]); characterize their associations with RHI exposure; and compare players who did or did not meet diagnostic criteria for traumatic encephalopathy syndrome (TES).
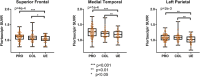
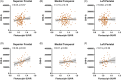
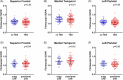
Results: Significantly elevated flortaucipir uptake was observed in former football players (PRO+COL) in prespecified regions (p < 0.05). Association between regional flortaucipir uptake and estimated cumulative head impact exposure was only observed in the superior frontal region in former players over 60 years old. Flortaucipir PET was not able to differentiate TES groups.
Discussion: Additional studies are needed to further understand tau pathology in CTE and other individuals with a history of RHI.
Trial registration: ClinicalTrials.gov NCT02798185.
Keywords: CTE; PET; Tau; flortaucipir; football.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
M.L.A. receives royalties or royalties from Oxford University Press Inc. and the Michael J. Fox Foundation. C.H.A. receives consulting fees from CND Life Science and owns stock or stock options from Cionic. L.J.B. is Editor in Chief, Journal of Neuro‐Ophthalmology, and is a Speaker for the Napa Eye Symposium. R.A. receives consulting fees from Signant Health, Biogen, Davos Alzheimer's Collaborative, travel support from Alzheimer's Drug Discovery Foundation and American Heart Association, and research support from Gates Ventures, Davos Alzheimer's Collaborative, and Linus Health. S.B. receives consulting fees from Boston University to participate in diagnostic consensus conferences for the DIAGNOSE CTE project, she also received payment from speakership at UC Irvine and UCLA, S.B. also participates on a Data Safety Monitoring Board/Advisory Board for the Cleveland Clinic. W.B.B. receives royalties or license fees from Springer Publishing, consulting fees for legal cases involving concussion & CTE, he also receives payments and travel support for speakership at American Academy of Clinical Neuropsychology (AACN) and National Academy of Neuropsychology. D.W.D. consults for Amgen, Atria, CapiThera Ltd., Cerecin, Ceruvia Lifesciences LLC, CoolTech, Ctrl M, Allergan, AbbVie, Biohaven, GlaxoSmithKline, Lundbeck, Eli Lilly, Novartis, Impel, Satsuma, Theranica, WL Gore, Genentech, Nocira, Perfood, Praxis, AYYA Biosciences, Revance, Pfizer; receives honoraria from American Academy of Neurology, Headache Cooperative of the Pacific, Canadian Headache Society, MF Med Ed Research, Biopharm Communications, CEA Group Holding Company (Clinical Education Alliance LLC), Teva (speaking), Amgen (speaking), Eli Lilly (speaking), Lundbeck (speaking), Pfizer (speaking), Vector Psychometric Group, Clinical Care Solutions, CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Academy for Continued Healthcare Learning, Majallin LLC, Medlogix Communications, Medica Communications LLC, MJH Lifesciences, Miller Medical Communications, WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, Cambridge University Press; has Non‐profit board membership at American Brain Foundation, American Migraine Foundation, ONE Neurology, Precon Health Foundation, International Headache Society Global Patient Advocacy Coalition, Atria Health Collaborative, Arizona Brain Injury Alliance, Domestic Violence HOPE Foundation/Panfila; receives research support from Department of Defense, National Institutes of Health, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, Henry Jackson Foundation, Patient Centered Outcomes Research Institute (PCORI); owns stock options of Aural analytics, Axon Therapeutics (shares/board), ExSano, Palion, Man and Science, Healint, Theranica, Second Opinion/Mobile Health, Epien, Nocira, King‐Devick Technologies, EigenLyfe, AYYA Biosciences, Cephalgia Group, Atria Health; owns shares and/or serves on board of Matterhorn, Ontologics, King‐Devick Technologies, Cephalgia Group. D.I.K. receives royalties from Springer/Demos Publishing for a textbook on brain injury, honorarium Brain Injury Association of MA for lectures on CTE, payment for expert testimony cases involving brain injury. J.M. receives travel support from Concussion Legacy Foundation. G.D.R. receives consulting fees from Eli Lilly, GE Healthcare, Genentech/Roche, Alector; payments from Efficient LLC, Associate Editor for JAMA Neurology, Miller Medical Communications, and Medscape, paid for participation on a Data Safety Monitoring Board or Advisory Board for Johnson & Johnson. K.J. receives Consulting fees from Novartis, Merck. Y.T. receives consulting fees from American Medical Association for Editorial Service. J.L.C. has ownership of Neuropsychiatric Inventory (NPI) copyright, has provided consultation to AB Science, Acadia, Alkahest, AlphaCognition, ALZPath, Annovis, AriBio, Artery, Avanir, Biogen, Biosplice, Cassava, Cerevel, Clinilabs, Cortexyme, Diadem, EIP Pharma, Eisai, GatehouseBio, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lexeo, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Otsuka, PharmacotrophiX, PRODEO, Prothena, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, Unlearn AI, Vaxxinity, VigilNeuro, Zai Laboratories pharmaceutical, assessment, and investment companies; owns stock or stock options of ADAMAS, Acumen, Alkahest, Alzheon, AnnovisBio, Behren Therapeutics, BIOasis, MedAvante, and United Neuroscience; served on Data Safety Monitoring or Advisory Board of Acadia, Biogen, Genentech, Grifols, Janssen, Karuna, Otsuka, reMYND, Roche, Signant Health; he is Chief Scientific Advisor – CNS Innovations, LLC. R.A.S. Receives royalties from Psychological Assessment Resources, Inc., consults for Biogen and Lundbeck, he is unpaid member of NFL Players Association Mackey‐White Health and Safety Committee, Court‐Appointed Member of NCAA Student‐Athlete Concussion Injury Litigation, Medical Scientific Committee; owns Stock Options of King‐Devick Technologies, Inc. E.M.R. is a compensated scientific advisor for Scientific Advisor, Alzheon, Aural Analytics Denali, Enigma, Retromer Therapeutics, Vaxxinity, a co‐founder of ALZPath. He is Chairman of the Board, Flinn Foundation, Chairman of the Board, Arizona Alzheimer's Consortium. The remaining authors have no relevant conflicts of interest to disclose. Author disclosures are available in the supporting information.
Figures