Single-center Experience With Upadacitinib for Adolescents With Refractory Inflammatory Bowel Disease
- PMID: 38134405
- PMCID: PMC12102471
- DOI: 10.1093/ibd/izad300
Single-center Experience With Upadacitinib for Adolescents With Refractory Inflammatory Bowel Disease
Abstract
Background: Upadacitinib (UPA) is a novel selective JAK inhibitor approved for adults with ulcerative colitis (UC) and with positive phase 3 data for Crohn's disease (CD). Pediatric off-label use is common due to delays in pediatric approvals; real-world data on UPA are needed to understand the safety and effectiveness in pediatric IBD.
Methods: This is a single-center retrospective case series study of adolescents (12-17 years) with inflammatory bowel disease IBD on UPA. The primary outcome was postinduction steroid-free clinical remission (SF-CR) defined as Pediatric UC Activity Index (PUCAI) or Pediatric CD Activity Index (PCDAI) ≤10. Secondary outcomes include postinduction clinical response (decrease ≥12.5 in PUCAI/PCDAI), postinduction C-reactive protein (CRP) normalization, 6-month SF-CR, and intestinal ultrasound response and remission. Adverse events were recorded through last follow-up.
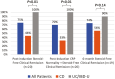
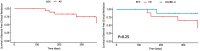
Results: Twenty patients (9 CD, 10 UC, 1 IBD-U; 55% female; median age 15 years, 90% ≥2 biologics) were treated with UPA for ≥12 weeks (median 51 [43-63] weeks). Upadacitinib was used as monotherapy in 55% and as combination with ustekinumab and vedolizumab in 35% and 10%, respectively. Week 12 SF-CR was achieved in 75% (15/20) and 80% (16/20) with CRP normalization. About 3/4 (14/19) achieved SF-CR at 6 months. Adverse event occurred in 2 patients (10%): Cytomegalovirus colitis requiring hospitalization and hyperlipidemia requiring no treatment. In the 75% with ultrasound monitoring, response and remission were achieved in 77% and 60%, respectively.
Conclusion: While awaiting pediatric registration trials, our data suggest that UPA is effective in inducing and maintaining SF-CR in adolescents with highly-refractory IBD with an acceptable safety profile.
Keywords: Crohn’s disease; adolescent; inflammatory bowel disease; pediatric; ulcerative colitis; upadacitinib.
Plain language summary
This case series presents novel data on the effectiveness and safety of upadacitinib in adolescent patients with IBD.
© The Author(s) 2023. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
S.B.: Support from the Digestive Disease Research Foundation (DDRF) Fellowship Program. M.C.D.: Consulting fees from Abbvie, Amgen, Arena Pharmaceuticals, AstraZeneca, Boehringer -Ingelheim, Celgene, Ferring, Genentech, Gilead, Hoffmann-La Roche, Janssen, Pfizer, Prometheus Biosciences, Takeda, and Target PharmaSolutions. Research funding from Abbvie, Janssen, Pfizer, Prometheus Biosciences, and Takeda. Cofounder and CEO of Trellus Health. M.T.D.: Consulting fees from Neurologica Corp., a subsidiary of Samsung Electronics Co., Ltd. A.K.: No conflict of interest. N.P.: No conflict of interest. D.D.: No conflict of interest. E.A.S.: Grant support from the NIH (K23DK125760-01).
Figures
References
-
- Crowley E, Ma C, Andic M, et al. Impact of drug approval pathways for paediatric inflammatory bowel disease. J Crohns Colitis 2022;16(2):331-335. - PubMed
-
- Burr NE, Gracie DJ, Black CJ, Ford AC. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis: systematic review and network meta-analysis. Gut. 2021;71(10):1976-1987. - PubMed
-
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. - PubMed
-
- D’Haens G, Panes J, Louis E, et al. Upadacitinib was efficacious and well-tolerated over 30 months in patients with Crohn’s disease in the CELEST extension study. Clin Gastroenterol Hepatol. 2022;20(10):2337-2346.e3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous