CHLOROPLAST UNUSUAL POSITIONING 1 is a plant-specific actin polymerization factor regulating chloroplast movement
- PMID: 38134410
- PMCID: PMC10980345
- DOI: 10.1093/plcell/koad320
CHLOROPLAST UNUSUAL POSITIONING 1 is a plant-specific actin polymerization factor regulating chloroplast movement
Abstract
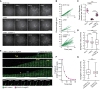
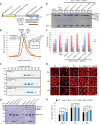
Plants have unique responses to fluctuating light conditions. One such response involves chloroplast photorelocation movement, which optimizes photosynthesis under weak light by the accumulation of chloroplasts along the periclinal side of the cell, which prevents photodamage under strong light by avoiding chloroplast positioning toward the anticlinal side of the cell. This light-responsive chloroplast movement relies on the reorganization of chloroplast actin (cp-actin) filaments. Previous studies have suggested that CHLOROPLAST UNUSUAL POSITIONING 1 (CHUP1) is essential for chloroplast photorelocation movement as a regulator of cp-actin filaments. In this study, we conducted comprehensive analyses to understand CHUP1 function. Functional, fluorescently tagged CHUP1 colocalized with and was coordinately reorganized with cp-actin filaments on the chloroplast outer envelope during chloroplast movement in Arabidopsis thaliana. CHUP1 distribution was reversibly regulated in a blue light- and phototropin-dependent manner. X-ray crystallography revealed that the CHUP1-C-terminal domain shares structural homology with the formin homology 2 (FH2) domain, despite lacking sequence similarity. Furthermore, the CHUP1-C-terminal domain promoted actin polymerization in the presence of profilin in vitro. Taken together, our findings indicate that CHUP1 is a plant-specific actin polymerization factor that has convergently evolved to assemble cp-actin filaments and enables chloroplast photorelocation movement.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures





References
-
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. . PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010:66(2):213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

