LRRC37B is a human modifier of voltage-gated sodium channels and axon excitability in cortical neurons
- PMID: 38134874
- PMCID: PMC10754148
- DOI: 10.1016/j.cell.2023.11.028
LRRC37B is a human modifier of voltage-gated sodium channels and axon excitability in cortical neurons
Abstract
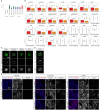
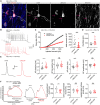
The enhanced cognitive abilities characterizing the human species result from specialized features of neurons and circuits. Here, we report that the hominid-specific gene LRRC37B encodes a receptor expressed in human cortical pyramidal neurons (CPNs) and selectively localized to the axon initial segment (AIS), the subcellular compartment triggering action potentials. Ectopic expression of LRRC37B in mouse CPNs in vivo leads to reduced intrinsic excitability, a distinctive feature of some classes of human CPNs. Molecularly, LRRC37B binds to the secreted ligand FGF13A and to the voltage-gated sodium channel (Nav) β-subunit SCN1B. LRRC37B concentrates inhibitory effects of FGF13A on Nav channel function, thereby reducing excitability, specifically at the AIS level. Electrophysiological recordings in adult human cortical slices reveal lower neuronal excitability in human CPNs expressing LRRC37B. LRRC37B thus acts as a species-specific modifier of human neuron excitability, linking human genome and cell evolution, with important implications for human brain function and diseases.
Keywords: FGF13; LRRC37; SCN1B; axon initial segment; cerebral cortex; gene duplicates; human brain evolution; neuronal excitability; voltage-gated channels.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.L.-P., P.V., J.d.W., A.L., K.W., J.S., F.R., N.L, and K.K. are inventors on a PCT application related to this work.
Figures













Comment in
-
A Human Touch: Hominid-Specific LRRC37B Regulates Axon Initial Segment Excitability.Epilepsy Curr. 2024 May 16;24(4):286-288. doi: 10.1177/15357597241253683. eCollection 2024 Jul-Aug. Epilepsy Curr. 2024. PMID: 39309051 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

