Structural evolution of fibril polymorphs during amyloid assembly
- PMID: 38134875
- PMCID: PMC7617692
- DOI: 10.1016/j.cell.2023.11.025
Structural evolution of fibril polymorphs during amyloid assembly
Abstract
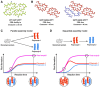
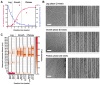
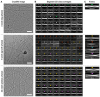
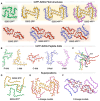
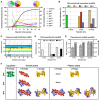
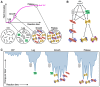
Cryoelectron microscopy (cryo-EM) has provided unprecedented insights into amyloid fibril structures, including those associated with disease. However, these structures represent the endpoints of long assembly processes, and their relationship to fibrils formed early in assembly is unknown. Consequently, whether different fibril architectures, with potentially different pathological properties, form during assembly remains unknown. Here, we used cryo-EM to determine structures of amyloid fibrils at different times during in vitro fibrillation of a disease-related variant of human islet amyloid polypeptide (IAPP-S20G). Strikingly, the fibrils formed in the lag, growth, and plateau phases have different structures, with new forms appearing and others disappearing as fibrillation proceeds. A time course with wild-type hIAPP also shows fibrils changing with time, suggesting that this is a general property of IAPP amyloid assembly. The observation of transiently populated fibril structures has implications for understanding amyloid assembly mechanisms with potential new insights into amyloid progression in disease.
Keywords: amyloid; amyloid polymorphism; cryoEM; diabetes; kinetics; protein aggregation; protein fibrils; protein structure; structural biology.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Schnabel J. Protein folding: the dark side of proteins. Nature. 2010;464:828–829. - PubMed
-
- Sergeeva AV, Galkin AP. Functional amyloids of eukaryotes: criteria, classification, and biological significance. Curr Genet. 2020;66:849–866. - PubMed
-
- Ferrone F. Analysis of protein aggregation kinetics. Methods Enzymol. 1999;309:256–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

