SP-R210 isoforms of Myosin18A modulate endosomal sorting and recognition of influenza A virus infection in macrophages
- PMID: 38135024
- PMCID: PMC10948314
- DOI: 10.1016/j.micinf.2023.105280
SP-R210 isoforms of Myosin18A modulate endosomal sorting and recognition of influenza A virus infection in macrophages
Abstract
Influenza A virus (IAV) infection causes acute and often lethal inflammation in the lung. The role of macrophages in this adverse inflammation is partially understood. The surfactant protein A receptor 210 (SP-R210) consists of two isoforms, a long (L) SP-R210L and a short (S) SP-R210S isoform encoded by alternative splicing of the myosin 18A gene. We reported that disruption of SP-R210L enhances cytosolic and endosomal antiviral response pathways. Here, we report that SP-R210L antagonizes type I interferon β (IFNβ), as depletion of SP-R210L potentiates IFNβ secretion. SP-R210 antibodies enhance and attenuate IFNβ secretion in SP-R210L replete and deficient macrophages, respectively, indicating that SP-R210 isoform stoichiometry alters macrophage function intrinsically. This reciprocal response is coupled to unopposed and restricted expression of viral genes in control and SP-R210L-deficient macrophages, respectively. Human monocytic cells with sub-stoichiometric expression of SP-R210L resist IAV infection, whereas alveolar macrophages with increased abundance of SP-R210L permit viral gene expression similar to murine macrophages. Uptake and membrane binding studies show that lack of SP-R210 isoforms does not impair IAV binding and internalization. Lack of SP-R210L, however, results in macropinocytic retention of the virus that depends on both SP-R210S and interferon-inducible transmembrane protein-3 (IFITM3). Mass spectrometry and Western blot analyses indicate that SP-R210 isoforms modulate differential recruitment of the Rho-family GTPase RAC1 and guanine nucleotide exchange factors. Our study suggests that SP-R210 isoforms modulate RAC-dependent macropinosomal sorting of IAV to discrete endosomal and lysosomal compartments that either permit or prevent endolysosomal escape and inflammatory sensing of viral genomes in macrophages.
Keywords: IFITM3; Influenza life-cycle; Macrophages; Macropinocytosis; Myosin 18A (MYO18A); Surfactant protein A receptor 210 (SP-R210).
Copyright © 2023 The Author(s). Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of competing interests
Author ZC is co-founder of Respana Therapeutic, Inc. (
Figures
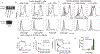





Similar articles
-
Genomic and epigenomic adaptation in SP-R210 (Myo18A) isoform-deficient macrophages.Immunobiology. 2021 Nov;226(6):152150. doi: 10.1016/j.imbio.2021.152150. Epub 2021 Oct 25. Immunobiology. 2021. PMID: 34735924 Free PMC article.
-
SP-R210 (Myo18A) Isoforms as Intrinsic Modulators of Macrophage Priming and Activation.PLoS One. 2015 May 12;10(5):e0126576. doi: 10.1371/journal.pone.0126576. eCollection 2015. PLoS One. 2015. PMID: 25965346 Free PMC article.
-
Disruption of the SP-A/SP-R210L (MYO18Aα) pathway prolongs gestation and reduces fetal survival during lipopolysaccharide-induced parturition in late gestation.Am J Physiol Lung Cell Mol Physiol. 2024 Apr 1;326(4):L508-L513. doi: 10.1152/ajplung.00383.2023. Epub 2024 Feb 13. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38349123 Free PMC article.
-
Emerging concepts of myosin 18A isoform mechanobiology in organismal and immune system physiology, development, and function.FASEB J. 2024 May 31;38(10):e23649. doi: 10.1096/fj.202400350R. FASEB J. 2024. PMID: 38776246 Review.
-
Roles of Glycans and Non-glycans on the Epithelium and in the Immune System in H1-H18 Influenza A Virus Infections.Methods Mol Biol. 2022;2556:205-242. doi: 10.1007/978-1-0716-2635-1_16. Methods Mol Biol. 2022. PMID: 36175637 Review.
Cited by
-
A ventilated perfused lung model platform to dissect the response of the lungs to viral infection.Trends Biotechnol. 2025 Jul;43(7):1714-1742. doi: 10.1016/j.tibtech.2025.03.012. Epub 2025 Apr 24. Trends Biotechnol. 2025. PMID: 40280814
-
Rho-GTPases subfamily: cellular defectors orchestrating viral infection.Cell Mol Biol Lett. 2025 May 2;30(1):55. doi: 10.1186/s11658-025-00722-w. Cell Mol Biol Lett. 2025. PMID: 40316910 Free PMC article. Review.
References
-
- Meischel T, Villalon-Letelier F, Saunders PM, Reading PC, Londrigan SL. Influenza A virus interactions with macrophages: Lessons from epithelial cells. Cellular microbiology 2020;22:e13170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

