Scalable generation of functional human iPSC-derived CAR-macrophages that efficiently eradicate CD19-positive leukemia
- PMID: 38135346
- PMCID: PMC10749073
- DOI: 10.1136/jitc-2023-007705
Scalable generation of functional human iPSC-derived CAR-macrophages that efficiently eradicate CD19-positive leukemia
Abstract
Background: Macrophages have recently become attractive therapeutics in cancer immunotherapy. The potential of macrophages to infiltrate and influence solid malignancies makes them promising targets for the chimeric antigen receptor (CAR) technology to redirect their stage of polarization, thus enhancing their anticancer capacities. Given the emerging interest for CAR-macrophages, generation of such cells so far mainly depends on peripheral blood monocytes, which are isolated from the respective donor prior to genetic manipulation. This procedure is time-intensive and cost-intensive, while, in some cases, insufficient monocyte amounts can be recovered from the donor, thus hampering the broad applicability of this technology. Hence, we demonstrate the generation and effectiveness of CAR-macrophages from various stem cell sources using also modern upscaling technologies for next generation immune cell farming.
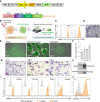
Methods: Primary human hematopoietic stem and progenitor cells and induced pluripotent stem cells were used to derive anti-CD19 CAR-macrophages. Anticancer activity of the cells was demonstrated in co-culture systems, including primary material from patients with leukemia. Generation of CAR-macrophages was facilitated by bioreactor technologies and single-cell RNA (scRNA) sequencing was used to characterize in-depth response and behavior of CAR-macrophages.
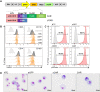
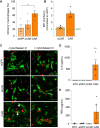
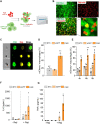
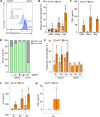
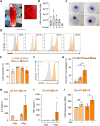
Results: Irrespective of the stem-cell source, CAR-macrophages exhibited enhanced and antigen-dependent phagocytosis of CD19+ target cancer cells with increased pro-inflammatory responses. Phagocytic capacity of CAR-macrophages was dependent on target cell CD19 expression levels with superior function of CAR-macrophages against CD19+ cancer cell lines and patient-derived acute lymphocytic leukemia cancer cells. scRNA sequencing revealed CAR-macrophages to be distinct from eGFP control cells after co-culture with target cells, which includes the activation of pro-inflammatory pathways and upregulation of chemokines and cytokines associated with adaptive immune cell recruitment, favoring the repolarization of CAR-macrophages to a pro-inflammatory state. Taken together, the data highlight the unique features of CAR-macrophages in combination with the successful upscaling of the production pipeline using a three-dimensional differentiation protocol and intermediate scale bioreactors.
Conclusion: In summary, our work provides insights into the seminal use and behavior of CAR-macrophages which are derived from various sources of stem cells, while introducing a unique technology for CAR-macrophage manufacturing, all dedicated to the clinical translation of CAR-macrophages within the field of anticancer immunotherapies.
Keywords: immunotherapy; macrophages; phagocytosis; receptors, chimeric antigen.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: NL, MA and TM have filled a patent application on the generation of human iPSC-derived macrophages. All other authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
