Effects of nintedanib on symptoms in patients with progressive pulmonary fibrosis
- PMID: 38135442
- PMCID: PMC10831140
- DOI: 10.1183/13993003.00752-2023
Effects of nintedanib on symptoms in patients with progressive pulmonary fibrosis
Abstract
Background: Dyspnoea and cough can have a profound impact on the lives of patients with pulmonary fibrosis. We investigated the effects of nintedanib on the symptoms and impact of pulmonary fibrosis in patients with progressive pulmonary fibrosis (PPF) in the INBUILD trial using the Living with Pulmonary Fibrosis (L-PF) questionnaire.
Methods: Patients had a fibrosing interstitial lung disease (ILD) (other than idiopathic pulmonary fibrosis) of >10% extent on high-resolution computed tomography (HRCT) and met criteria for ILD progression within the prior 24 months. Patients were randomised 1:1 to receive nintedanib or placebo. Changes in L-PF questionnaire scores from baseline to week 52 were assessed using mixed models for repeated measures.
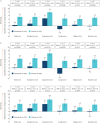
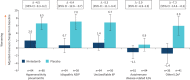
Results: In total, 663 patients were treated. Compared with placebo, there were significantly smaller increases (worsenings) in adjusted mean L-PF questionnaire total (0.5 versus 5.1), symptoms (1.3 versus 5.3), dyspnoea (4.3 versus 7.8) and fatigue (0.7 versus 4.0) scores in the nintedanib group at week 52. L-PF questionnaire cough score decreased in the nintedanib group and increased in the placebo group (-1.8 versus 4.3). L-PF questionnaire impacts score decreased slightly in the nintedanib group and increased in the placebo group (-0.2 versus 4.6). Similar findings were observed in patients with a usual interstitial pneumonia-like fibrotic pattern on HRCT and in patients with other fibrotic patterns on HRCT.
Conclusion: Based on changes in L-PF questionnaire scores, nintedanib reduced worsening of dyspnoea, fatigue and cough and the impacts of ILD over 52 weeks in patients with PPF.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: M. Wijsenbeek reports grants to her institution from The Dutch Pulmonary Fibrosis Patients Association, The Dutch Lung Foundation, The Netherlands Organisation for Health Research and Development, The Thorax Foundation, Sarcoidosis.nl, AstraZeneca/DaiichiSankyo, BI and Hoffmann-La Roche, and consulting or speaker fees from AstraZeneca, BI, Bristol Myers Squibb, CSL Behring, Galapagos, Galecto, Hoffmann-La Roche, Horizon Therapeutics, Kinevant Sciences, Molecure, NeRRe, Novartis, PureTech, Thyron, Trevi and Vicore. J.J. Swigris reports consulting fees from BI, Bristol Myers Squibb, CSL Behring and Tvardi; he is an unpaid member of the Board of Directors for Live Fully, Inc. and he is on the Medical Advisory Board for patientMpower and PF Warriors. Y. Inoue reports grants from the Japanese Ministry of Health, Labour, and Welfare, and the Japan Agency for Medical Research and Development, payment for presentations from BI, Kyorin, Shionogi, GlaxoSmithKline and Thermo Fisher, and has served as a consultant or steering committee member for BI, Galapagos, Roche, Taiho, CSL Behring, Vicore Pharma and Savara. M. Kreuter reports grants, consulting fees and fees for speaking from BI and Roche, and holds leadership or fiduciary roles with Deutsche gesellschaft für Pneumologiex, the European Respiratory Society and the German Respiratory Society. T.M. Maher reports consulting fees from AstraZeneca, Bayer, Blade Therapeutics, BI, Bristol Myers Squibb, Galapagos, Galecto, GlaxoSmithKline, IQVIA, Pliant, Respivant, Roche/Genentech, Theravance and Veracyte, and payment for presentations from BI and Roche/Genentech. T. Suda reports fees for speaking from BI. M. Baldwin, H. Mueller and K.B. Rohr are employees of BI. K.R. Flaherty reports grants paid to his institution from BI, royalties or licenses from UpToDate, and consulting fees from Arrowhead, AstraZeneca, Bellerophon, CSL Behring, Daewoong, DevPro, Dispersol, FibroGen, Horizon Therapeutics, Immunet, Insilico, Lupin, NeRRe, Pliant, Polarean, Pure Health, PureTech, Respivant, Roche/Genentech, Shionogi, Sun Pharmaceuticals, Trevi, United Therapeutics and Vicore.
Figures
Comment in
-
Nintedanib and symptoms of fibrotic lung disease: a glimmer of hope for patients living with pulmonary fibrosis.Eur Respir J. 2024 Feb 1;63(2):2400067. doi: 10.1183/13993003.00067-2024. Print 2024 Feb. Eur Respir J. 2024. PMID: 38302183 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
