Interplay between hippocampal TACR3 and systemic testosterone in regulating anxiety-associated synaptic plasticity
- PMID: 38135756
- PMCID: PMC11153148
- DOI: 10.1038/s41380-023-02361-z
Interplay between hippocampal TACR3 and systemic testosterone in regulating anxiety-associated synaptic plasticity
Abstract
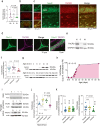
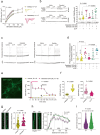
Tachykinin receptor 3 (TACR3) is a member of the tachykinin receptor family and falls within the rhodopsin subfamily. As a G protein-coupled receptor, it responds to neurokinin B (NKB), its high-affinity ligand. Dysfunctional TACR3 has been associated with pubertal failure and anxiety, yet the mechanisms underlying this remain unclear. Hence, we have investigated the relationship between TACR3 expression, anxiety, sex hormones, and synaptic plasticity in a rat model, which indicated that severe anxiety is linked to dampened TACR3 expression in the ventral hippocampus. TACR3 expression in female rats fluctuates during the estrous cycle, reflecting sensitivity to sex hormones. Indeed, in males, sexual development is associated with a substantial increase in hippocampal TACR3 expression, coinciding with elevated serum testosterone and a significant reduction in anxiety. TACR3 is predominantly expressed in the cell membrane, including the presynaptic compartment, and its modulation significantly influences synaptic activity. Inhibition of TACR3 activity provokes hyperactivation of CaMKII and enhanced AMPA receptor phosphorylation, associated with an increase in spine density. Using a multielectrode array, stronger cross-correlation of firing was evident among neurons following TACR3 inhibition, indicating enhanced connectivity. Deficient TACR3 activity in rats led to lower serum testosterone levels, as well as increased spine density and impaired long-term potentiation (LTP) in the dentate gyrus. Remarkably, aberrant expression of functional TACR3 in spines results in spine shrinkage and pruning, while expression of defective TACR3 increases spine density, size, and the magnitude of cross-correlation. The firing pattern in response to LTP induction was inadequate in neurons expressing defective TACR3, which could be rectified by treatment with testosterone. In conclusion, our study provides valuable insights into the intricate interplay between TACR3, sex hormones, anxiety, and synaptic plasticity. These findings highlight potential targets for therapeutic interventions to alleviate anxiety in individuals with TACR3 dysfunction and the implications of TACR3 in anxiety-related neural changes provide an avenue for future research in the field.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12:452–66. - PubMed
-
- Nathan BM, Palmert MR. Regulation and disorders of pubertal timing. J Endocrinol Metab Clin. 2005;34:617–41. - PubMed
-
- Aydogan U, Aydogdu A, Akbulut H, Sonmez A, Yuksel S, Basaran Y, et al. Increased frequency of anxiety, depression, quality of life and sexual life in young hypogonadotropic hypogonadal males and impacts of testosterone replacement therapy on these conditions. Endocr J. 2012;59:1099–105. - PubMed
-
- Rey RA, Grinspon R, Gottlieb S, Pasqualini T, Knoblovits P, Aszpis S, et al. Male hypogonadism: an extended classification based on a developmental, endocrine physiology‐based approach. J Androl. 2013;1:3–16. - PubMed
-
- Dwyer AA, Quinton R. Anatomy and physiology of the hypothalamic-pituitary-gonadal (HPG) axis. Adv Pract Endocrinol Nurs. 2019;43:839–52.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

