Comprehensive Exploration of the Growth and Lipid Synthesis Phases of T. oleaginosus Cultures Implementing Design of Experiments and Response Surface Methodology
- PMID: 38135950
- PMCID: PMC10741121
- DOI: 10.3390/bioengineering10121359
Comprehensive Exploration of the Growth and Lipid Synthesis Phases of T. oleaginosus Cultures Implementing Design of Experiments and Response Surface Methodology
Abstract
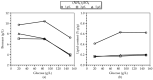
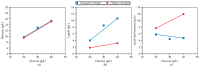
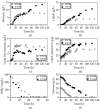
Trichosporon oleaginosus is an unconventional oleaginous yeast distinguished by its remarkable capacity to accumulate lipids in excess of 70% of its dry weight, particularly when cultivated in nitrogen-restricted conditions with ample carbon sources. A pivotal question that arises pertains to the nutrient dynamics in the culture medium, which give rise to both the excessive lipid content and corresponding lipid concentration. While previous research has predominantly focused on evaluating the impact of the initial carbon-to-nitrogen (C/N) ratio on lipid production, the precise critical thresholds of glucose and ammonium sulfate ((NH4)2SO4) at which growth and intracellular lipid production are either stimulated or impeded remain inadequately defined. This study employs an experimental design and response surface methodology to investigate the complex mechanism of lipid accumulation and its interaction with cellular growth. Application of the aforementioned methodologies resulted in the production of 10.6 g/L of microbial oil in batch cultures under conditions that correspond to a C/N ratio of 76. However, the primary objective is to generate knowledge to facilitate the development of efficient fed-batch cultivation strategies that optimize lipid production exclusively employing inorganic nitrogen sources by finely adjusting carbon and nitrogen levels. The intricate interaction between these levels is comprehensively addressed in the present study, while it is additionally revealed that as glucose levels rise within a non-inhibitory range, lipid-free biomass production decreases while lipid accumulation simultaneously increases. These findings set the stage for further exploration and the potential development of two-stage cultivation approaches, aiming to fully decouple growth and lipid production. This advancement holds the promise of bringing microbial oil production closer to commercial viability.
Keywords: C/N ratio parameterization; Cryptococcus curvatus; design of experiments; inorganic nitrogen source; lipogenesis; media composition optimization; microbial lipids; oleaginous yeast; response surface methodology (RSM).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Crognale S., Liuzzi F., D’Annibale A., De Bari I., Petruccioli M. Cynara Cardunculus a Novel Substrate for Solid-State Production of Aspergillus Tubingensis Cellulases and Sugar Hydrolysates. Biomass Bioenergy. 2019;127:105276. doi: 10.1016/j.biombioe.2019.105276. - DOI
-
- Zhang X., Yan S., Tyagi R.D., Surampalli R.Y., Valéro J.R. Wastewater Sludge as Raw Material for Microbial Oils Production. Appl. Energy. 2014;135:192–201. doi: 10.1016/j.apenergy.2014.08.078. - DOI
-
- Lamichhane Upadhyaya K., Mondala A., Hernandez R., French T., Green M., McFarland L., Holmes W. Biocrude Production by Activated Sludge Microbial Cultures Using Pulp and Paper Wastewaters as Fermentation Substrate. Environ. Technol. 2013;34:2171–2178. doi: 10.1080/09593330.2013.808246. - DOI - PubMed
LinkOut - more resources
Full Text Sources

