Osteogenic Effect of a Bioactive Calcium Alkali Phosphate Bone Substitute in Humans
- PMID: 38135999
- PMCID: PMC10741049
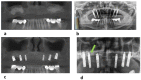
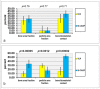
- DOI: 10.3390/bioengineering10121408
Osteogenic Effect of a Bioactive Calcium Alkali Phosphate Bone Substitute in Humans
Abstract
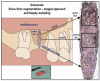
(1) Background: The desire to avoid autograft harvesting in implant dentistry has prompted an ever-increasing quest for bioceramic bone substitutes, which stimulate osteogenesis while resorbing in a timely fashion. Consequently, a highly bioactive silicon containing calcium alkali orthophosphate (Si-CAP) material was created, which previously was shown to induce greater bone cell maturation and bone neo-formation than β-tricalcium phosphate (β-TCP) in vivo as well as in vitro. Our study tested the hypothesis that the enhanced effect on bone cell function in vitro and in sheep in vivo would lead to more copious bone neoformation in patients following sinus floor augmentation (SFA) employing Si-CAP when compared to β-TCP. (2) Methods: The effects of Si-CAP on osteogenesis and Si-CAP resorbability were evaluated in biopsies harvested from 38 patients six months after SFA in comparison to β-TCP employing undecalcified histology, histomorphometry, and immunohistochemical analysis of osteogenic marker expression. (3) Results: Si-CAP as well as β-TCP supported matrix mineralization and bone formation. Apically furthest away from the original bone tissue, Si-CAP induced significantly higher bone formation, bone-bonding (bone-bioceramic contact), and granule resorption than β-TCP. This was in conjunction with a higher expression of osteogenic markers. (4) Conclusions: Si-CAP induced higher and more advanced bone formation and resorbability than β-TCP, while β-TCP's remarkable osteoconductivity has been widely demonstrated. Hence, Si-CAP constitutes a well-suited bioactive graft choice for SFA in the clinical arena.
Keywords: bioactive bone grafting material; bioactivity; bioceramics; bone regeneration; calcium alkali orthophosphate materials; osteogenesis; silicon release; sinus floor augmentation.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Szabó G., Huys L., Coulthard P., Maiorana C., Garagiola U., Barabás J., Németh Z., Hrabák K., Suba Z. A prospective multicenter randomized clinical trial of autogenous bone versus beta-tricalcium phosphate graft alone for bilateral sinus elevation: Histologic and histomorphometric evaluation. Int. J. Oral. Maxillofac. Implant. 2005;20:371–3781. - PubMed
-
- Knabe C., Stiller M., Adel-Khattab D., Ducheyne P. Dental graft materials. In: Ducheyne P., Healy K., Hutmacher D., Grainger D.W., Kirkpatrick J.P., editors. Comprehensive Biomaterials II. Volume 7. Elsevier; Oxford, UK: 2017. pp. 378–405. Chapter 7.20.
-
- Knabe C., Mele A., Peleska B., Kann P.H., Adel-Khattab D., Renz H., Reuss A., Bohner M., Stiller M. Effect of sex-hormone levels, sex, body mass index and other host factors on human craniofacial bone regeneration with bioactive tricalcium phosphate grafts. Biomaterials. 2017;123:48–62. doi: 10.1016/j.biomaterials.2017.01.035. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

