Anti-Apoptosis Therapy for Meniscal Avascular Zone Repair: A Proof-of-Concept Study in a Lapine Model
- PMID: 38136013
- PMCID: PMC10740472
- DOI: 10.3390/bioengineering10121422
Anti-Apoptosis Therapy for Meniscal Avascular Zone Repair: A Proof-of-Concept Study in a Lapine Model
Abstract
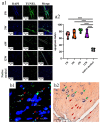
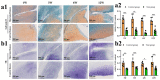
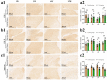
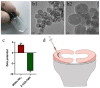
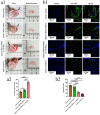
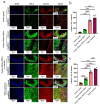
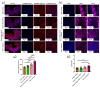
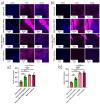
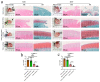
In the present study, 24 rabbits were firstly used to evaluate the apoptosis index and matrix degeneration after untreated adult meniscal tears. Vertical tears (0.25 cm in length) were prepared in the avascular zone of the anterior horn. Specimens were harvested at 1, 3, 6, 12 weeks postoperatively. The apoptosis index around tear sites stayed at a high level throughout the whole follow-up period. The depletion of glycosaminoglycans (GAG) and aggrecan at the tear site was observed, while the deposition of COL I and COL II was not affected, even at the last follow-up of 12 weeks after operation. The expression of SOX9 decreased significantly; no cellularity was observed at the wound interface at all timepoints. Secondly, another 20 rabbits were included to evaluate the effects of anti-apoptosis therapy on rescuing meniscal cells and enhancing meniscus repair. Longitudinal vertical tears (0.5 cm in length) were made in the meniscal avascular body. Tears were repaired by the inside-out suture technique, or repaired with sutures in addition to fibrin gel and blank silica nanoparticles, or silica nanoparticles encapsulating apoptosis inhibitors (z-vad-fmk). Samples were harvested at 12 months postoperatively. We found the locally administered z-vad-fmk agent at the wound interface significantly alleviated meniscal cell apoptosis and matrix degradation, and enhanced meniscal repair in the avascular zone at 12 months after operation. Thus, local administration of caspase inhibitors (z-vad-fmk) is a promising therapeutic strategy for alleviating meniscal cell loss and enhancing meniscal repair after adult meniscal tears in the avascular zone.
Keywords: apoptosis; caspases inhibitor; meniscal repair; meniscal tear; z-vad-fmk.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Logerstedt D.S., Scalzitti D.A., Bennell K.L., Hinman R.S., Silvers-Granelli H., Ebert J., Hambly K., Carey J.L., Snyder-Mackler L., Axe M.J., et al. Knee Pain and Mobility Impairments: Meniscal and Articular Cartilage Lesions Revision 2018. J. Orthop. Sports Phys. Ther. 2018;48:A1–A50. doi: 10.2519/jospt.2018.0301. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

