Extracellular Vesicles and Their Renin-Angiotensin Cargo as a Link between Metabolic Syndrome and Parkinson's Disease
- PMID: 38136165
- PMCID: PMC10741149
- DOI: 10.3390/antiox12122045
Extracellular Vesicles and Their Renin-Angiotensin Cargo as a Link between Metabolic Syndrome and Parkinson's Disease
Abstract
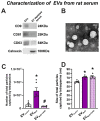
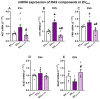
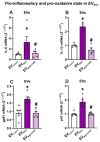
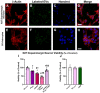
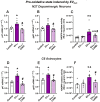
Several studies showed an association between metabolic syndrome (MetS) and Parkinson's disease (PD). The linking mechanisms remain unclear. MetS promotes low-grade peripheral oxidative stress and inflammation and dysregulation of the adipose renin-angiotensin system (RAS). Interestingly, brain RAS dysregulation is involved in the progression of dopaminergic degeneration and PD. Circulating extracellular vesicles (EVs) from MetS fat tissue can cross the brain-blood barrier and may act as linking signals. We isolated and characterized EVs from MetS and control rats and analyzed their mRNA and protein cargo using RT-PCR and the ExoView R200 platform, respectively. Furthermore, cultures of the N27 dopaminergic cell line and the C6 astrocytic cell line were treated with EVs from MetS rats. EVs were highly increased in MetS rat serum, which was inhibited by treatment of the rats with the angiotensin type-1-receptor blocker candesartan. Furthermore, EVs from MetS rats showed increased pro-oxidative/pro-inflammatory and decreased anti-oxidative/anti-inflammatory RAS components, which were inhibited in candesartan-treated MetS rats. In cultures, EVs from MetS rats increased N27 cell death and modulated C6 cell function, upregulating markers of neuroinflammation and oxidative stress, which were inhibited by the pre-treatment of cultures with candesartan. The results from rat models suggest EVs and their RAS cargo as a mechanism linking Mets and PD.
Keywords: NADPH-oxidase; adipocytes; angiotensin receptor blockers; exosomes; neurodegeneration; neuroinflammation; obesity; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Calabrese V., Santoro A., Monti D., Crupi R., Di Paola R., Latteri S., Cuzzocrea S., Zappia M., Giordano J., Calabrese E.J., et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018;115:80–91. doi: 10.1016/j.freeradbiomed.2017.10.379. - DOI - PubMed
-
- Kusters C.D.J., Paul K.C., Duarte Folle A., Keener A.M., Bronstein J.M., Bertram L., Hansen J., Horvath S., Sinsheimer J.S., Lill C.M., et al. Increased Menopausal Age Reduces the Risk of Parkinson’s Disease: A Mendelian Randomization Approach. Mov. Disord. 2021;36:2264–2272. doi: 10.1002/mds.28760. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

