Contribution of Mitochondrial Reactive Oxygen Species to Chronic Hypoxia-Induced Pulmonary Hypertension
- PMID: 38136180
- PMCID: PMC10741244
- DOI: 10.3390/antiox12122060
Contribution of Mitochondrial Reactive Oxygen Species to Chronic Hypoxia-Induced Pulmonary Hypertension
Abstract
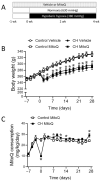
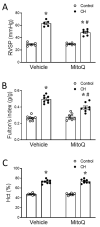
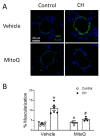
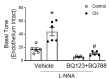
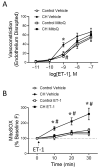
Pulmonary hypertension (PH) resulting from chronic hypoxia (CH) occurs in patients with chronic obstructive pulmonary diseases, sleep apnea, and restrictive lung diseases, as well as in residents at high altitude. Previous studies from our group and others demonstrate a detrimental role of reactive oxygen species (ROS) in the pathogenesis of CH-induced PH, although the subcellular sources of ROS are not fully understood. We hypothesized that mitochondria-derived ROS (mtROS) contribute to enhanced vasoconstrictor reactivity and PH following CH. To test the hypothesis, we exposed rats to 4 weeks of hypobaric hypoxia (PB ≈ 380 mmHg), with control rats housed in ambient air (PB ≈ 630 mmHg). Chronic oral administration of the mitochondria-targeted antioxidant MitoQ attenuated CH-induced decreases in pulmonary artery (PA) acceleration time, increases in right ventricular systolic pressure, right ventricular hypertrophy, and pulmonary arterial remodeling. In addition, endothelium-intact PAs from CH rats exhibited a significantly greater basal tone compared to those from control animals, as was eliminated via MitoQ. CH also augmented the basal tone in endothelium-disrupted PAs, a response associated with increased mtROS production in primary PA smooth muscle cells (PASMCs) from CH rats. However, we further uncovered an effect of NO synthase inhibition with Nω-nitro-L-arginine (L-NNA) to unmask a potent endothelial vasoconstrictor influence that accentuates mtROS-dependent vasoconstriction following CH. This basal tone augmentation in the presence of L-NNA disappeared following combined endothelin A and B receptor blockade with BQ123 and BQ788. The effects of using CH to augment vasoconstriction and PASMC mtROS production in exogenous endothelin 1 (ET-1) were similarly prevented by MitoQ. We conclude that mtROS participate in the development of CH-induced PH. Furthermore, mtROS signaling in PASMCs is centrally involved in enhanced pulmonary arterial constriction following CH, a response potentiated by endogenous ET-1.
Keywords: hypoxia; mitochondria; pulmonary hypertension; reactive oxygen species; vasoconstriction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Galiè N., Humbert M., Vachiery J.-L., Gibbs S., Lang I., Torbicki A., Simonneau G., Peacock A., Vonk Noordegraaf A., Beghetti M., et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. - DOI - PubMed
-
- Shimoda L.A., Sham J.S., Sylvester J.T. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol. Res. 2000;49:549–560. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

