Portulaca Oleracea L. (Purslane) Extract Protects Endothelial Function by Reducing Endoplasmic Reticulum Stress and Oxidative Stress through AMPK Activation in Diabetic Obese Mice
- PMID: 38136251
- PMCID: PMC10741183
- DOI: 10.3390/antiox12122132
Portulaca Oleracea L. (Purslane) Extract Protects Endothelial Function by Reducing Endoplasmic Reticulum Stress and Oxidative Stress through AMPK Activation in Diabetic Obese Mice
Abstract
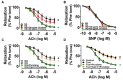
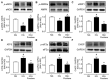
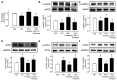
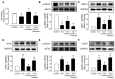
Portulaca oleracea L. (purslane) is a food and a traditional drug worldwide. It exhibits anti-inflammatory, anti-oxidative, anti-tumor, and anti-diabetic bioactivities; but its activity on diabetic-associated endothelial dysfunction is unknown. This study aimed to investigate the effect of purslane on endothelial function and the underlying mechanisms. Male C57BL/6 mice had 14-week ad libitum access to a high-fat rodent diet containing 60% kcal% fat to induce obesity and diabetes whereas purslane extract (200 mg/kg/day) was administered during the last 4 weeks via intragastric gavage. Primary rat aortic endothelial cells and isolated mouse aortas were cultured with a risk factor, high glucose or tunicamycin, together with purslane extract. By ESI-QTOF-MS/MS, flavonoids and their glycoside products were identified in the purslane extract. Exposure to high glucose or tunicamycin impaired acetylcholine-induced endothelium-dependent relaxations in aortas and induced endoplasmic reticulum (ER) stress and oxidative stress with the downregulation of 5' AMP-activated protein kinase (AMPK)/ endothelial nitric oxide synthase (eNOS) signaling. Co-incubation with purslane significantly ameliorated these impairments. The effects of purslane were abolished by Compound C (AMPK inhibitor). Four-week purslane treatment ameliorated aortic relaxations, ER stress, and oxidative stress in diabetic obese mice. This study supported that purslane protected endothelial function, and inhibited ER stress and oxidative stress in vasculature through AMPK/eNOS activation, revealing its therapeutic potential against vascular complications in diabetes.
Keywords: diabetes mellitus; endoplasmic reticulum stress; endothelial function; nitric oxide; purslane; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

