Telomere Maintenance Mechanisms in a Cohort of High-Risk Neuroblastoma Tumors and Its Relation to Genomic Variants in the TERT and ATRX Genes
- PMID: 38136279
- PMCID: PMC10741428
- DOI: 10.3390/cancers15245732
Telomere Maintenance Mechanisms in a Cohort of High-Risk Neuroblastoma Tumors and Its Relation to Genomic Variants in the TERT and ATRX Genes
Abstract
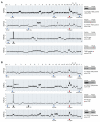
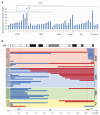
Tumor cells are hallmarked by their capacity to undergo unlimited cell divisions, commonly accomplished either by mechanisms that activate TERT or through the alternative lengthening of telomeres pathway. Neuroblastoma is a heterogeneous pediatric cancer, and the aim of this study was to characterize telomere maintenance mechanisms in a high-risk neuroblastoma cohort. All tumor samples were profiled with SNP microarrays and, when material was available, subjected to whole genome sequencing (WGS). Telomere length was estimated from WGS data, samples were assayed for the ALT biomarker c-circles, and selected samples were subjected to methylation array analysis. Samples with ATRX aberration in this study were positive for c-circles, whereas samples with either MYCN amplification or TERT re-arrangement were negative for c-circles. Both ATRX aberrations and TERT re-arrangement were enriched in 11q-deleted samples. An association between older age at diagnosis and 1q-deletion was found in the ALT-positive group. TERT was frequently placed in juxtaposition to a previously established gene in neuroblastoma tumorigenesis or cancer in general. Given the importance of high-risk neuroblastoma, means for mitigating active telomere maintenance must be therapeutically explored.
Keywords: ALT; ATRX; TERT; TMM; neuroblastoma; pediatric cancer; telomeres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wu X.M., Tang W.R., Luo Y. [ALT--alternative lengthening of telomere] Yi Chuan. 2009;31:1185–1191. - PubMed
-
- Caren H., Kryh H., Nethander M., Sjoberg R.M., Trager C., Nilsson S., Abrahamsson J., Kogner P., Martinsson T. High-risk neuroblastoma tumors with 11q-deletion display a poor prognostic, chromosome instability phenotype with later onset. Proc. Natl. Acad. Sci. USA. 2010;107:4323–4328. doi: 10.1073/pnas.0910684107. - DOI - PMC - PubMed
Grants and funding
- 20-1213/the Swedish Cancer Society
- 19-566/the Swedish Cancer Society
- 19-139/the Swedish Childhood Cancer Foundation
- 22-156/the Swedish Childhood Cancer Foundation
- 17-122/the Swedish Childhood Cancer Foundation
- 18-99/the Swedish Childhood Cancer Foundation
- ALFGBG-447171/the Swedish state under the LUA/ALF agreement
- RB13-0204/the Swedish Foundation for Strategic Research
- SF/Sahlgrenska University hospital and Laboratory medicine research and development grant
- KAW 2018.0057/the Knut and Alice Wallenberg foundation
LinkOut - more resources
Full Text Sources

