Relevance of Molecular Pathology for the Diagnosis of Sex Cord-Stromal Tumors of the Ovary: A Narrative Review
- PMID: 38136408
- PMCID: PMC10741682
- DOI: 10.3390/cancers15245864
Relevance of Molecular Pathology for the Diagnosis of Sex Cord-Stromal Tumors of the Ovary: A Narrative Review
Abstract
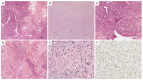
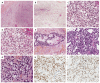
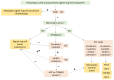
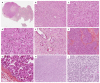
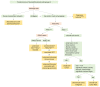
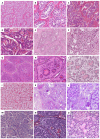
Ovarian sex cord-stromal tumors (SCSTs) account for 8% of all primary ovarian neo-plasms. Accurate diagnosis is crucial since each subtype has a specific prognostic and treatment. Apart from fibrosarcomas, stromal tumors are benign while sex cord tumors may recur, sometimes with a significant time to relapse. Although the diagnosis based on morphology is straightforward, in some cases the distinction between stromal tumors and sex cord tumors may be tricky. Indeed, the immunophenotype is usually nonspecific between stromal tumors and sex cord tumors. Therefore, molecular pathology plays an important role in the diagnosis of such entities, with pathognomonic or recurrent alterations, such as FOXL2 variants in adult granulosa cell tumors. In addition, these neoplasms may be associated with genetic syndromes, such as Peutz-Jeghers syndrome for sex cord tumors with annular tubules, and DICER1 syndrome for Sertoli-Leydig cell tumors (SLCTs), for which the pathologist may be in the front line of syndromic suspicion. Molecular pathology of SCST is also relevant for patient prognosis and management. For instance, the DICER1 variant is associated with moderately to poorly differentiated SLCTS and a poorer prognosis. The present review summarizes the histomolecular criteria useful for the diagnosis of SCST, using recent molecular data from the literature.
Keywords: DICER1; FOXL2; Sertoli–Leydig cell tumor; diagnostic algorithm; granulosa cell tumor; molecular diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Siegmund S., Sholl L.M., Cornejo K.M., Sangoi A.R., Otis C.N., Mehra R., Hirsch M.S., Acosta A.M. Molecular assessment of testicular adult granulosa cell tumor demonstrates significant differences when compared to ovarian counterparts. Mod. Pathol. 2022;35:697–704. doi: 10.1038/s41379-021-00977-6. - DOI - PubMed
-
- Collins K., Sholl L.M., Vargas S.O., Cornejo K.M., Kravstov O., Dickson B.C., Idrees M.T., Ulbright T.M., Acosta A.M. Testicular Juvenile Granulosa Cell Tumors Demonstrate Recurrent Loss of Chromosome 10 and Absence of Molecular Alterations Described in Ovarian Counterparts. Mod. Pathol. 2023;36:100142. doi: 10.1016/j.modpat.2023.100142. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

