Acid Ceramidase Inhibitor LCL-805 Antagonizes Akt Signaling and Promotes Iron-Dependent Cell Death in Acute Myeloid Leukemia
- PMID: 38136410
- PMCID: PMC10742122
- DOI: 10.3390/cancers15245866
Acid Ceramidase Inhibitor LCL-805 Antagonizes Akt Signaling and Promotes Iron-Dependent Cell Death in Acute Myeloid Leukemia
Abstract
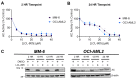
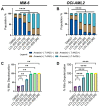
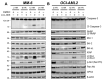
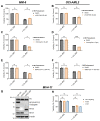
Acute myeloid leukemia (AML) is an aggressive hematologic malignancy requiring urgent treatment advancements. Ceramide is a cell-death-promoting signaling lipid that plays a central role in therapy-induced cell death. We previously determined that acid ceramidase (AC), a ceramide-depleting enzyme, is overexpressed in AML and promotes leukemic survival and drug resistance. The ceramidase inhibitor B-13 and next-generation lysosomal-localizing derivatives termed dimethylglycine (DMG)-B-13 prodrugs have been developed but remain untested in AML. Here, we report the in vitro anti-leukemic efficacy and mechanism of DMG-B-13 prodrug LCL-805 across AML cell lines and primary patient samples. LCL-805 inhibited AC enzymatic activity, increased total ceramides, and reduced sphingosine levels. A median EC50 value of 11.7 μM was achieved for LCL-805 in cell viability assays across 32 human AML cell lines. As a single agent tested across a panel of 71 primary AML patient samples, a median EC50 value of 15.8 μM was achieved. Exogenous ceramide supplementation with C6-ceramide nanoliposomes, which is entering phase I/II clinical trial for relapsed/refractory AML, significantly enhanced LCL-805 killing. Mechanistically, LCL-805 antagonized Akt signaling and led to iron-dependent cell death distinct from canonical ferroptosis. These findings elucidated key factors involved in LCL-805 cytotoxicity and demonstrated the potency of combining AC inhibition with exogenous ceramide.
Keywords: Akt; C6-ceramide nanoliposome; LCL-805; acid ceramidase; acute myeloid leukemia; ceramide; dimethylglycine-B-13 prodrug; iron chelation; sphingolipids.
Conflict of interest statement
D.J.F. has received research funding, honoraria, and/or stock options from AstraZeneca, Dren Bio, Recludix Pharma, and Kymera Therapeutics. T.P.L. has received Scientific Advisory Board membership, consultancy fees, honoraria, and/or stock options from Keystone Nano, Flagship Labs 86, Dren Bio, Recludix Pharma, Kymera Therapeutics, and Prime Genomics. M.C.C. owns shares in Keystone Nano. There are no conflicts of interest with the work presented in this manuscript. Other authors declare no competing interests. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
Figures




Update of
-
Acid Ceramidase Inhibitor LCL-805 Antagonizes Akt Signaling and Promotes Iron-Dependent Cell Death in Acute Myeloid Leukemia.bioRxiv [Preprint]. 2023 Oct 23:2023.10.21.563437. doi: 10.1101/2023.10.21.563437. bioRxiv. 2023. Update in: Cancers (Basel). 2023 Dec 15;15(24):5866. doi: 10.3390/cancers15245866. PMID: 37961314 Free PMC article. Updated. Preprint.
References
-
- Sasaki K., Ravandi F., Kadia T.M., DiNardo C.D., Short N.J., Borthakur G., Jabbour E., Kantarjian H.M. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer. 2021;127:2049–2061. doi: 10.1002/cncr.33458. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

