Transgenerational Epigenetic DNA Methylation Editing and Human Disease
- PMID: 38136557
- PMCID: PMC10742326
- DOI: 10.3390/biom13121684
Transgenerational Epigenetic DNA Methylation Editing and Human Disease
Abstract
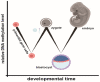
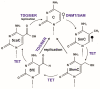
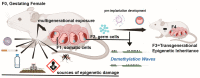
During gestation, maternal (F0), embryonic (F1), and migrating primordial germ cell (F2) genomes can be simultaneously exposed to environmental influences. Accumulating evidence suggests that operating epi- or above the genetic DNA sequence, covalent DNA methylation (DNAme) can be recorded onto DNA in response to environmental insults, some sites which escape normal germline erasure. These appear to intrinsically regulate future disease propensity, even transgenerationally. Thus, an organism's genome can undergo epigenetic adjustment based on environmental influences experienced by prior generations. During the earliest stages of mammalian development, the three-dimensional presentation of the genome is dramatically changed, and DNAme is removed genome wide. Why, then, do some pathological DNAme patterns appear to be heritable? Are these correctable? In the following sections, I review concepts of transgenerational epigenetics and recent work towards programming transgenerational DNAme. A framework for editing heritable DNAme and challenges are discussed, and ethics in human research is introduced.
Keywords: DNA methylation; cytosine; dCas; development; epigenetic editing; epigenetics; epimutation; germline; heritable; transgenerational.
Conflict of interest statement
The author declares no conflict of interest.
Figures




Similar articles
-
Transgenerational impact of grand-paternal lifetime exposures to both folic acid deficiency and supplementation on genome-wide DNA methylation in male germ cells.Andrology. 2023 Jul;11(5):927-942. doi: 10.1111/andr.13399. Epub 2023 Feb 17. Andrology. 2023. PMID: 36697378
-
Transgenerational inheritance: how impacts to the epigenetic and genetic information of parents affect offspring health.Hum Reprod Update. 2019 Sep 11;25(5):518-540. doi: 10.1093/humupd/dmz017. Hum Reprod Update. 2019. PMID: 31374565
-
Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line.PLoS One. 2013 Jul 15;8(7):e66318. doi: 10.1371/journal.pone.0066318. Print 2013. PLoS One. 2013. PMID: 23869203 Free PMC article.
-
Distinctions between transgenerational and non-transgenerational epimutations.Mol Cell Endocrinol. 2014 Dec;398(1-2):13-23. doi: 10.1016/j.mce.2014.07.016. Epub 2014 Jul 28. Mol Cell Endocrinol. 2014. PMID: 25079508 Review.
-
Erasure of DNA methylation in rat fetal germ cells is sex-specific and sensitive to maternal high-fat diet.J Dev Orig Health Dis. 2024 Sep 26;15:e19. doi: 10.1017/S2040174424000230. J Dev Orig Health Dis. 2024. PMID: 39324180
Cited by
-
Overview and Prospects of DNA Sequence Visualization.Int J Mol Sci. 2025 Jan 8;26(2):477. doi: 10.3390/ijms26020477. Int J Mol Sci. 2025. PMID: 39859192 Free PMC article. Review.
References
-
- Tompkins J.D., Riggs A.D. An Epigenetic Perspective on the Failing Heart and Pluripotent-Derived-Cardiomyocytes for Cell Replacement Therapy. Front. Biol. 2015;10:11–27. doi: 10.1007/s11515-014-1340-0. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

