Adipokines and Bacterial Metabolites: A Pivotal Molecular Bridge Linking Obesity and Gut Microbiota Dysbiosis to Target
- PMID: 38136564
- PMCID: PMC10742113
- DOI: 10.3390/biom13121692
Adipokines and Bacterial Metabolites: A Pivotal Molecular Bridge Linking Obesity and Gut Microbiota Dysbiosis to Target
Abstract
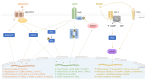
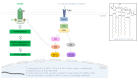
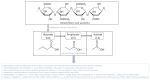
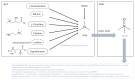
Adipokines are essential mediators produced by adipose tissue and exert multiple biological functions. In particular, adiponectin, leptin, resistin, IL-6, MCP-1 and PAI-1 play specific roles in the crosstalk between adipose tissue and other organs involved in metabolic, immune and vascular health. During obesity, adipokine imbalance occurs and leads to a low-grade pro-inflammatory status, promoting insulin resistance-related diabetes and its vascular complications. A causal link between obesity and gut microbiota dysbiosis has been demonstrated. The deregulation of gut bacteria communities characterizing this dysbiosis influences the synthesis of bacterial substances including lipopolysaccharides and specific metabolites, generated via the degradation of dietary components, such as short-chain fatty acids, trimethylamine metabolized into trimethylamine-oxide in the liver and indole derivatives. Emerging evidence suggests that these bacterial metabolites modulate signaling pathways involved in adipokine production and action. This review summarizes the current knowledge about the molecular links between gut bacteria-derived metabolites and adipokine imbalance in obesity, and emphasizes their roles in key pathological mechanisms related to oxidative stress, inflammation, insulin resistance and vascular disorder. Given this interaction between adipokines and bacterial metabolites, the review highlights their relevance (i) as complementary clinical biomarkers to better explore the metabolic, inflammatory and vascular complications during obesity and gut microbiota dysbiosis, and (ii) as targets for new antioxidant, anti-inflammatory and prebiotic triple action strategies.
Keywords: adipokines; bacterial metabolites; gut microbiota dysbiosis; insulin resistance; obesity; vascular disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO . World Health Statistics 2018: Monitoring Health for the SDGs, Sustainable Development Goals. World Health Organization; Geneva, Switzerland: 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

