Kinetics of Phosphate Ions and Phytase Activity Production for Lactic Acid-Producing Bacteria Utilizing Milling and Whitening Stages Rice Bran as Biopolymer Substrates
- PMID: 38136641
- PMCID: PMC10741578
- DOI: 10.3390/biom13121770
Kinetics of Phosphate Ions and Phytase Activity Production for Lactic Acid-Producing Bacteria Utilizing Milling and Whitening Stages Rice Bran as Biopolymer Substrates
Abstract
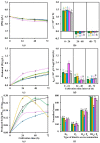
A study evaluated nine kinetic data and four kinetic parameters related to growth, production of various phytase activities (PEact), and released phosphate ion concentration ([Pi]) from five lactic acid bacteria (LAB) strains cultivated in three types of media: phytate (IP6), milling stage rice bran (MsRB), and whitening stage rice bran (WsRB). Score ranking techniques were used, combining these kinetic data and parameters to select the most suitable LAB strain for each medium across three cultivation time periods (24, 48, and 72 h). In the IP6 medium, Lacticaseibacillus casei TISTR 1500 exhibited statistically significant highest (p ≤ 0.05) normalized summation scores using a 2:1 weighting between kinetic and parameter data sets. This strain also had the statistically highest levels (p ≤ 0.05) of produced phosphate ion concentration ([Pi]) (0.55 g/L) at 72 h and produced extracellular specific phytase activity (ExSp-PEact) (0.278 U/mgprotein) at 48 h. For the MsRB and WsRB media, Lactiplantibacillus plantarum TISTR 877 performed exceptionally well after 72 h of cultivation. It produced ([Pi], ExSp-PEact) pairs of (0.53 g/L, 0.0790 U/mgprotein) in MsRB and (0.85 g/L, 0.0593 U/mgprotein) in WsRB, respectively. Overall, these findings indicate the most promising LAB strains for each medium and cultivation time based on their ability to produce phosphate ions and extracellular specific phytase activity. The selection process utilized a combination of kinetic data and parameter analysis.
Keywords: lactic acid bacteria; phytic acid; rice bran; solid waste; sustainability.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Seesuriyachan P., Kuntiya A., Kawee-ai A., Techapun C., Chaiyaso T., Leksawasdi N. Improvement in efficiency of lignin degradation by Fenton reaction using synergistic catalytic action. Ecol. Eng. 2015;85:283–287. doi: 10.1016/j.ecoleng.2015.10.013. - DOI
-
- Nunta R., Techapun C., Jantanasakulwong K., Chaiyaso T., Seesuriyachan P., Khemacheewakul J., Mahakuntha C., Porninta K., Sommanee S., Trinh N.T., et al. Batch and continuous cultivation processes of Candida tropicalis TISTR 5306 for ethanol and pyruvate decarboxylase production in fresh longan juice with optimal carbon to nitrogen molar ratio. J. Food Process Eng. 2019;42:e13227. doi: 10.1111/jfpe.13227. - DOI
-
- Nunta R., Techapun C., Kuntiya A., Hanmuangjai P., Moukamnerd C., Khemacheewakul J., Sommanee S., Reungsang A., Boonmee Kongkeitkajorn M., Leksawasdi N. Ethanol and phenylacetylcarbinol production processes of Candida tropicalis TISTR 5306 and Saccharomyces cerevisiae TISTR 5606 in fresh juices from longan fruit of various sizes. J. Food Process. Preserv. 2018;42:e13815. doi: 10.1111/jfpp.13815. - DOI
-
- Wattanapanom S., Muenseema J., Techapun C., Jantanasakulwong K., Sanguanchaipaiwong V., Chaiyaso T., Hanmoungjai P., Seesuriyachan P., Khemacheewakul J., Nunta R., et al. Kinetic parameters of Candida tropicalis TISTR 5306 for ethanol production process using an optimal enzymatic digestion strategy of assorted grade longan solid waste powder. Chiang Mai. J. Sci. 2019;46:1036–1054.
-
- Bodie A.R., Micciche A.C., Atungulu G.G., Rothrock M.J., Jr., Ricke S.C. Current trends of rice milling byproducts for agricultural applications and alternative food production systems. Front. Sustain. Food Syst. 2019;3:47. doi: 10.3389/fsufs.2019.00047. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

