Bioengineering the Antimicrobial Activity of Yeast by Recombinant Thanatin Production
- PMID: 38136753
- PMCID: PMC10741026
- DOI: 10.3390/antibiotics12121719
Bioengineering the Antimicrobial Activity of Yeast by Recombinant Thanatin Production
Abstract
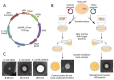
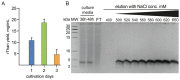
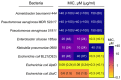
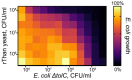
The global spread of antibiotic resistance marks the end of the era of conventional antibiotics. Mankind desires new molecular tools to fight pathogenic bacteria. In this regard, the development of new antimicrobials based on antimicrobial peptides (AMPs) is again of particular interest. AMPs have various mechanisms of action on bacterial cells. Moreover, AMPs have been reported to be efficient in preclinical studies, demonstrating a low level of resistance formation. Thanatin is a small, beta-hairpin antimicrobial peptide with a bacterial-specific mode of action, predetermining its low cytotoxicity toward eukaryotic cells. This makes thanatin an exceptional candidate for new antibiotic development. Here, a microorganism was bioengineered to produce an antimicrobial agent, providing novel opportunities in antibiotic research through the directed creation of biocontrol agents. The constitutive heterologous production of recombinant thanatin (rThan) in the yeast Pichia pastoris endows the latter with antibacterial properties. Optimized expression and purification conditions enable a high production level, yielding up to 20 mg/L of rThan from the culture medium. rThan shows a wide spectrum of activity against pathogenic bacteria, similarly to its chemically synthesized analogue. The designed approach provides new avenues for AMP engineering and creating live biocontrol agents to fight antibiotic resistance.
Keywords: antibiotic resistance; antimicrobial peptides; recombinant antibiotics; thanatin; yeast biocontrol agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Xie J. Grand Challenge of Antibiotics Resistance: A Global, Multidisciplinary Effort Is Needed. Front. Antibiot. 2022;1:984076. doi: 10.3389/frabi.2022.984076. - DOI
-
- de Breij A., Riool M., Cordfunke R.A., Malanovic N., de Boer L., Koning R.I., Ravensbergen E., Franken M., van der Heijde T., Boekema B.K., et al. The Antimicrobial Peptide SAAP-148 Combats Drug-Resistant Bacteria and Biofilms. Sci. Transl. Med. 2018;10:eaan4044. doi: 10.1126/scitranslmed.aan4044. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

