Characterization of Inflammasomes and Their Regulation in the Red Fox
- PMID: 38136879
- PMCID: PMC10741141
- DOI: 10.3390/ani13243842
Characterization of Inflammasomes and Their Regulation in the Red Fox
Abstract
Background: Inflammasomes recognize endogenous and exogenous danger signals, and subsequently induce the secretion of IL-1β. Studying inflammasomes in the red fox (Vulpes vulpes) is crucial for wildlife veterinary medicine, as it can help control inflammatory diseases in foxes.
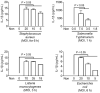
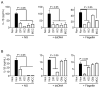
Methods: We investigated the activation and intracellular mechanisms of three inflammasomes (NLRP3, AIM2, and NLRC4) in fox peripheral blood mononuclear cells (PBMCs), using established triggers and inhibitors derived from humans and mice.
Results: Fox PBMCs exhibited normal activation and induction of IL-1β secretion in response to representative inflammasome triggers (ATP and nigericin for NLRP3, dsDNA for AIM2, flagellin for NLRC4). Additionally, PBMCs showed normal IL-1β secretion when inoculated with inflammasome-activating bacteria. In inhibitors of the inflammasome signaling pathway, fox inflammasome activation was compared with mouse inflammasomes. MCC950, a selective NLRP3 inhibitor, suppressed the secretion of dsDNA- and flagellin-mediated IL-1β in foxes, unlike mice.
Conclusions: These findings suggest that NLRP3 may have a common role in dsDNA- and flagellin-mediated inflammasome activation in the red fox. It implies that this fox inflammasome biology can be applied to the treatment of inflammasome-mediated diseases in the red fox.
Keywords: Vulpes vulpes; cytokine; inflammasome; interleukin-1beta; red foxes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

