The Influence of Arsenic Co-Exposure in a Model of Alcohol-Induced Neurodegeneration in C57BL/6J Mice
- PMID: 38137081
- PMCID: PMC10741530
- DOI: 10.3390/brainsci13121633
The Influence of Arsenic Co-Exposure in a Model of Alcohol-Induced Neurodegeneration in C57BL/6J Mice
Abstract
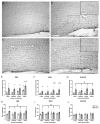
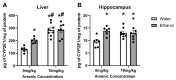
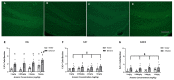
Both excessive alcohol consumption and exposure to high levels of arsenic can lead to neurodegeneration, especially in the hippocampus. Co-exposure to arsenic and alcohol can occur because an individual with an Alcohol Use Disorder (AUD) is exposed to arsenic in their drinking water or food or because of arsenic found directly in alcoholic beverages. This study aims to determine if co-exposure to alcohol and arsenic leads to worse outcomes in neurodegeneration and associated mechanisms that could lead to cell death. To study this, mice were exposed to a 10-day gavage model of alcohol-induced neurodegeneration with varying doses of arsenic (0, 0.005, 2.5, or 10 mg/kg). The following were examined after the last dose of ethanol: (1) microglia activation assessed via immunohistochemical detection of Iba-1, (2) reactive oxygen and nitrogen species (ROS/RNS) using a colorimetric assay, (3) neurodegeneration using Fluoro-Jade® C staining (FJC), and 4) arsenic absorption using ICP-MS. After exposure, there was an additive effect of the highest dose of arsenic (10 mg/kg) in the dentate gyrus of alcohol-induced FJC+ cells. This additional cell loss may have been due to the observed increase in microglial reactivity or increased arsenic absorption following co-exposure to ethanol and arsenic. The data also showed that arsenic caused an increase in CYP2E1 expression and ROS/RNS production in the hippocampus which could have independently contributed to increased neurodegeneration. Altogether, these findings suggest a potential cyclical impact of co-exposure to arsenic and ethanol as ethanol increases arsenic absorption but arsenic also enhances alcohol's deleterious effects in the CNS.
Keywords: CYP2E1; alcohol-related brain damage; arsenic toxicity; hippocampus; microglial activation; neuroimmune.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


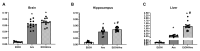
References
-
- GBD 2016 Alcohol and Drug Use Collaborators The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5:987–1012. doi: 10.1016/S2215-0366(18)30337-7. - DOI - PMC - PubMed
-
- Nielsen S.J., Kit B.K., Fakhouri T., Ogden C.L. Calories consumed from alcoholic beverages by U.S. adults, 2007–2010. NCHS Data Brief. 2012;110:1–8. - PubMed
Grants and funding
- 2019-AARGD-642198/ALZ/Alzheimer's Association/United States
- U54 AA019765/AA/NIAAA NIH HHS/United States
- R25 MH129791/MH/NIMH NIH HHS/United States
- SC1GM139696/GM/NIGMS NIH HHS/United States
- U54 AA030451/AA/NIAAA NIH HHS/United States
- U54 AA030463/AA/NIAAA NIH HHS/United States
- P30 ES025128/ES/NIEHS NIH HHS/United States
- U01 AA019925/AA/NIAAA NIH HHS/United States
- U54AA030451, U54AA030463, U01AA019925),/AA/NIAAA NIH HHS/United States
- SC1 GM139696/GM/NIGMS NIH HHS/United States
- S025128/ES/NIEHS NIH HHS/United States
- R25MH129791/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources

