Rescuing Infected Deep Brain Stimulation Therapies in Severely Affected Patients
- PMID: 38137098
- PMCID: PMC10742038
- DOI: 10.3390/brainsci13121650
Rescuing Infected Deep Brain Stimulation Therapies in Severely Affected Patients
Abstract
(1) Background: Infections in deep brain stimulation (DBS) hardware, while an undesired complication of DBS surgeries, can be effectively addressed. Minor infections are typically treated with wound revision and IV antibiotics. However, when visible hardware infection occurs, most centers opt for complete removal, leaving the patient in a preoperative state and necessitating post-removal care. To avoid the need for such care, a novel technique was developed. (2) Methods: The electrodes are placed at the exact same spot and then led to the contralateral side. new extensions and a new generator contralateral to the infection as well. Subsequently, the infected system is removed. This case series includes six patients. (3) Results: The average duration of DBS system implantation before the second surgery was 272 days. Only one system had to be removed after 18 months due to reoccurring infection; the others remained unaffected. Laboratory alterations and pathogens were identified in only half of the patients. (4) Conclusions: The described surgical technique proves to be safe, well tolerated, and serves as a viable alternative to complete system removal. Importantly, it effectively prevents the need of post-removal care for patients.
Keywords: deep brain stimulation; infection; salvage surgery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
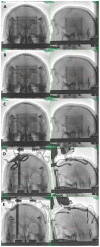
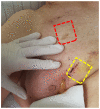
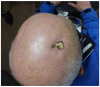

References
-
- Abode-Iyamah K.O., Chiang H.-Y., Woodroffe R.W., Park B., Jareczek F.J., Nagahama Y., Winslow N., Herwaldt L.A., Greenlee J.D.W. Deep brain stimulation hardware-related infections: 10-year experience at a single institution. J. Neurosurg. 2018;130:629–638. doi: 10.3171/2017.9.JNS1780. - DOI - PMC - PubMed
-
- Jakobs M., Helmers A.-K., Synowitz M., Slotty P.J., Anthofer J.M., Schlaier J.R., Kloss M., Unterberg A.W., Kiening K.L. A multicenter, open-label, controlled trial on acceptance, convenience, and complications of rechargeable internal pulse generators for deep brain stimulation: The Multi Recharge Trial. J. Neurosurg. 2019;133:821–829. doi: 10.3171/2019.5.JNS19360. - DOI - PubMed
LinkOut - more resources
Full Text Sources

