Dupilumab-Associated Blepharoconjunctivitis: Clinical and Morphological Aspects
- PMID: 38137325
- PMCID: PMC10740631
- DOI: 10.3390/biomedicines11123104
Dupilumab-Associated Blepharoconjunctivitis: Clinical and Morphological Aspects
Abstract
Purpose: To describe the clinical and morphologic changes in the ocular surface microstructure of patients affected with moderate-to-severe Atopic Dermatitis (AD) before and during Dupilumab treatment.
Methods: This is a monocentric observational study on thirty-three patients affected with AD before and during Dupilumab treatment. All patients underwent a slit-lamp examination: complete clinical assessment, Break Up Time test (BUT), Schirmer test, and corneal staining grading (Oxford scale) were performed. Meibomian Glands Dysfunction (MGD) evaluation (Meibography), Non-invasive Keratograph Break Up Time test (NIKBUT), Tear Meniscus Height (TMH), and ocular Redness Score (RS) have been investigated using an OCULUS Keratograph. In vivo images of the conjunctiva, cornea, and meibomian glands have been acquired by confocal microscopy.
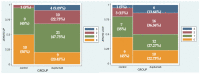
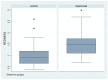
Results: Sixty-six eyes were included in our study: twenty-two eyes of 11 naive patients with indication for treatment but not in therapy yet (Group 1) and forty-four eyes of 22 patients treated with Dupilumab for at least 4 months (subcutaneous administration of 300 mg every 2 weeks) (Group 2). Either patients treated with Dupilumab or naive patients with moderate-to-severe forms of AD had a tear film instability (TBUT and NIKBUT reduced), whereas the quantity of the tear film was overall normal (Schirmer test and TMH), without statistically significant differences between the two groups. When Meibography was performed with the Keratograph, the difference between Group 1 and Group 2 was statistically significant in terms of Meiboscore (p = 0.0043 and p = 0.0242, respectively), as well as the difference in terms of mean RS. These results paired well with the confocal microscopy results in which we found a decrease in the goblet cell population in the conjunctival epithelium in the treated group (5.2 cells/mm), along with inflammatory cells that were more concentrated around the adenoid lumina of the meibomian glands.
Conclusions: In recent years, the use of Dupilumab has been increasing, but mild-to-severe conjunctivitis is a common side effect. Our major results demonstrate a loss of meibomian glands at the Keratograph examination: we can assume a reduced meibum secretion and an evaporative dry eye with MGD. We suggest that the inflammation of the ocular surface may involve not only the cornea and the conjunctiva, but also the meibomian glands, and Dupilumab may play a role. However, the frequency of clear conjunctivitis is not as common as reported in the literature.
Keywords: Dupilumab; atopic dermatitis; cornea; meibomian glands.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Kolb L., Ferrer-Bruker S.J. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Atopic Dermatitis. - PubMed
-
- Blauvelt A., de Bruin-Weller M., Gooderham M., Cather J.C., Weisman J., Pariser D., Simpson E.L., Papp K.A., Hong H.C., Rubel D., et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303. doi: 10.1016/S0140-6736(17)31191-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources

