Frequency and Pattern of Worldwide Ocular Gene Therapy Clinical Trials up to 2022
- PMID: 38137345
- PMCID: PMC10740821
- DOI: 10.3390/biomedicines11123124
Frequency and Pattern of Worldwide Ocular Gene Therapy Clinical Trials up to 2022
Abstract
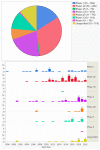
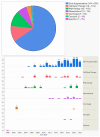
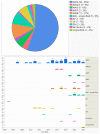
The purpose of this study is to describe worldwide gene therapy clinical trials aimed at treating ophthalmic disorders. Information regarding all worldwide clinical trials was collected through 15 different sources, including ClinicalTrials.gov. There were 159 gene therapy clinical trials on ophthalmic diseases up until 2022. Phase 1/2 trials had the highest frequency (50-32%), followed by phase 2 (33-21%); 107 trials (67%) were conducted in a single country, and 50 trials (31%) were multinational. Overall, the USA was the site of 113 (71%) single or multinational trials. Of the trials, 153 (96%) targeted retina and optic nerve disorders, 3 (2%) glaucoma, 2 (1%) uveitis, and 1 (1%) cornea; 104 trials (65%) employed gene augmentation using viral vectors, and the remaining employed other methods such as inhibitory RNA (18-11%) and cell-based gene therapy using encapsulated cell technology (18-11%). For gene augmentation trials, adeno-associated virus was used for transgene delivery in 87% of cases. The most common conditions targeted by gene augmentation included inherited retinal (74%) and age-related macular degeneration (wet, 14%; dry, 7%). Overall, a large number of gene therapy clinical trials have been conducted in the eye, and so far, one has led to regulatory approval.
Keywords: Leber congenital amaurosis; age-related macular degeneration; clinical trials; gene augmentation; gene therapy; inherited retinal diseases; ocular; ophthalmic; retinitis pigmentosa; viral vectors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rosenberg S.A., Aebersold P., Cornetta K., Kasid A., Morgan R.A., Moen R., Karson E.M., Lotze M.T., Yang J.C., Topalian S.L. Gene Transfer into Humans—Immunotherapy of Patients with Advanced Melanoma, Using Tumor-Infiltrating Lymphocytes Modified by Retroviral Gene Transduction. N. Engl. J. Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

